β-Catenin-Dependent Wnt Signaling: A Pathway in Acute Cutaneous Wounding
- PMID: 29481398
- PMCID: PMC6545117
- DOI: 10.1097/PRS.0000000000004170
β-Catenin-Dependent Wnt Signaling: A Pathway in Acute Cutaneous Wounding
Retraction in
-
β-Catenin-Dependent Wnt Signaling: A Pathway in Acute Cutaneous Wounding: Retraction.Plast Reconstr Surg. 2018 Oct;142(4):1107. doi: 10.1097/PRS.0000000000005012. Plast Reconstr Surg. 2018. PMID: 30252825 Free PMC article. No abstract available.
Abstract
Background: Acute wound healing is a dynamic process that results in the formation of scar tissue. The mechanisms of this process are not well understood; numerous signaling pathways are thought to play a major role. Here, the authors have identified β-catenin-dependent Wnt signaling as an early acute-phase reactant in acute wound healing and scar formation.
Methods: The authors created 6-mm full-thickness excisional cutaneous wounds on adult β-catenin-dependent Wnt signal (BAT-gal) reporter mice. The expression of canonical Wnt after wounding was analyzed using X-gal staining and quantitative real-time polymerase chain reaction. Next, recombinant mouse Wnt3a (rmWnt3a) was injected subcutaneously to the wound edge, daily. The mice were killed at stratified time points, up to 15 days after injury. Histologic analysis, quantitative real-time polymerase chain reaction, and Western blot were performed.
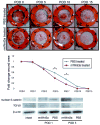
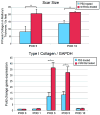
Results: Numerous individual Wnt ligands increased in expression after wounding, including Wnt3a, Wnt4, Wnt10a, and Wnt11. A specific pattern of Wnt activity was observed, localized to the hair follicle and epidermis. Mice injected with rmWnt3a exhibited faster wound closure, increased scar size, and greater expression of fibroblast growth factor receptor-2 and type I collagen.
Conclusions: The authors' data suggest that β-catenin-dependent Wnt signaling expression increases shortly after cutaneous wounding, and exogenous rmWnt3a accelerates reepithelialization, wound matrix maturation, and scar formation. Future experiments will focus on the intersection of Wnt signaling and other known profibrotic cytokines.
Figures



References
-
- Cheon SS, Wei Q, Gurung A, et al. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20(6):692–701. - PubMed
-
- Okuse T, Chiba T, Katsuumi I, Imai K. Differential expression and localization of WNTs in an animal model of skin wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2005;13(5):491–497. - PubMed
-
- Labus MB, Stirk CM, Thompson WD, Melvin WT. Expression of Wnt genes in early wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 1998;6(1):58–64. - PubMed
-
- Colwell AS, Krummel TM, Longaker MT, Lorenz HP. Wnt-4 expression is increased in fibroblasts after TGF-beta1 stimulation and during fetal and postnatal wound repair. Plastic and reconstructive surgery. 2006;117(7):2297–2301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

