Evaluation of the Trypanosoma brucei 6-oxopurine salvage pathway as a potential target for drug discovery
- PMID: 29481567
- PMCID: PMC5843355
- DOI: 10.1371/journal.pntd.0006301
Evaluation of the Trypanosoma brucei 6-oxopurine salvage pathway as a potential target for drug discovery
Abstract
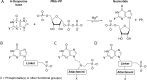
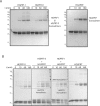
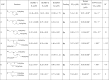
Due to toxicity and compliance issues and the emergence of resistance to current medications new drugs for the treatment of Human African Trypanosomiasis are needed. A potential approach to developing novel anti-trypanosomal drugs is by inhibition of the 6-oxopurine salvage pathways which synthesise the nucleoside monophosphates required for DNA/RNA production. This is in view of the fact that trypanosomes lack the machinery for de novo synthesis of the purine ring. To provide validation for this approach as a drug target, we have RNAi silenced the three 6-oxopurine phosphoribosyltransferase (PRTase) isoforms in the infectious stage of Trypanosoma brucei demonstrating that the combined activity of these enzymes is critical for the parasites' viability. Furthermore, we have determined crystal structures of two of these isoforms in complex with several acyclic nucleoside phosphonates (ANPs), a class of compound previously shown to inhibit 6-oxopurine PRTases from several species including Plasmodium falciparum. The most potent of these compounds have Ki values as low as 60 nM, and IC50 values in cell based assays as low as 4 μM. This data provides a solid platform for further investigations into the use of this pathway as a target for anti-trypanosomal drug discovery.
Conflict of interest statement
Part of the research carried out at IOCB by DH and PS was supported by fund of Gilead Sciences and IOCB Research Centre (GSRC) supported by Gilead Sciences, Inc. Aza-ANPs are subject of joined patent of IOCB and UQ (WO2013166545).
Figures









Similar articles
-
Crystal structures and inhibition of Trypanosoma brucei hypoxanthine-guanine phosphoribosyltransferase.Sci Rep. 2016 Oct 27;6:35894. doi: 10.1038/srep35894. Sci Rep. 2016. PMID: 27786284 Free PMC article.
-
Stereo-Defined Acyclic Nucleoside Phosphonates are Selective and Potent Inhibitors of Parasite 6-Oxopurine Phosphoribosyltransferases.J Med Chem. 2022 Mar 10;65(5):4030-4057. doi: 10.1021/acs.jmedchem.1c01881. Epub 2022 Feb 17. J Med Chem. 2022. PMID: 35175749
-
Acyclic nucleoside phosphonates as possible chemotherapeutics against Trypanosoma brucei.Drug Discov Today. 2020 Jun;25(6):1043-1053. doi: 10.1016/j.drudis.2020.02.008. Epub 2020 Mar 2. Drug Discov Today. 2020. PMID: 32135205 Review.
-
Synthesis of branched 9-[2-(2-phosphonoethoxy)ethyl]purines as a new class of acyclic nucleoside phosphonates which inhibit Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase.Bioorg Med Chem. 2009 Sep 1;17(17):6218-32. doi: 10.1016/j.bmc.2009.07.044. Epub 2009 Jul 25. Bioorg Med Chem. 2009. PMID: 19666228
-
6-oxopurine phosphoribosyltransferase: a target for the development of antimalarial drugs.Curr Top Med Chem. 2011;11(16):2085-102. doi: 10.2174/156802611796575911. Curr Top Med Chem. 2011. PMID: 21619515 Review.
Cited by
-
In silico Metabolic Pathway Analysis Identifying Target Against Leishmaniasis - A Kinetic Modeling Approach.Front Genet. 2020 Mar 6;11:179. doi: 10.3389/fgene.2020.00179. eCollection 2020. Front Genet. 2020. PMID: 32211028 Free PMC article.
-
Kinetic and Structural Characterization of Trypanosoma cruzi Hypoxanthine-Guanine-Xanthine Phosphoribosyltransferases and Repurposing of Transition-State Analogue Inhibitors.Biochemistry. 2023 Jul 18;62(14):2182-2201. doi: 10.1021/acs.biochem.3c00116. Epub 2023 Jul 7. Biochemistry. 2023. PMID: 37418678 Free PMC article.
-
Acyclic nucleoside phosphonates with adenine nucleobase inhibit Trypanosoma brucei adenine phosphoribosyltransferase in vitro.Sci Rep. 2021 Jun 25;11(1):13317. doi: 10.1038/s41598-021-91747-6. Sci Rep. 2021. PMID: 34172767 Free PMC article.
-
Inosine triphosphate pyrophosphatase from Trypanosoma brucei cleanses cytosolic pools from deaminated nucleotides.Sci Rep. 2022 Apr 18;12(1):6408. doi: 10.1038/s41598-022-10149-4. Sci Rep. 2022. PMID: 35436992 Free PMC article.
-
Kinetic Characterization and Inhibition of Trypanosoma cruzi Hypoxanthine-Guanine Phosphoribosyltransferases.Biochemistry. 2022 Oct 4;61(19):2088-2105. doi: 10.1021/acs.biochem.2c00312. Epub 2022 Sep 15. Biochemistry. 2022. PMID: 36193631 Free PMC article.
References
-
- Simarro PP, Cecchi G, Franco JR, Paone M, Diarra A, et al. (2012) Estimating and mapping the population at risk of sleeping sickness. PLoS Negl Trop Dis 6: e1859 doi: 10.1371/journal.pntd.0001859 - DOI - PMC - PubMed
-
- Simarro PP, Diarra A, Ruiz Postigo JA, Franco JR, Jannin JG (2011) The human African trypanosomiasis control and surveillance programme of the World Health Organization 2000–2009: the way forward. PLoS Negl Trop Dis 5: e1007 doi: 10.1371/journal.pntd.0001007 - DOI - PMC - PubMed
-
- WHO (2013) Control and surveillance of human African trypanosomiasis. World Health Organ Tech Rep Ser 984: 1–237. - PubMed
-
- Nagle AS, Khare S, Kumar AB, Supek F, Buchynskyy A, et al. (2014) Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis. Chem Rev 114: 11305–11347. doi: 10.1021/cr500365f - DOI - PMC - PubMed
-
- Graf FE, Ludin P, Arquint C, Schmidt RS, Schaub N, et al. (2016) Comparative genomics of drug resistance in Trypanosoma brucei rhodesiense. Cell Mol Life Sci 73: 3387–3400. doi: 10.1007/s00018-016-2173-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

