Value encoding in the globus pallidus: fMRI reveals an interaction effect between reward and dopamine drive
- PMID: 29481966
- PMCID: PMC5929903
- DOI: 10.1016/j.neuroimage.2018.02.048
Value encoding in the globus pallidus: fMRI reveals an interaction effect between reward and dopamine drive
Abstract
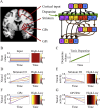
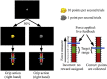
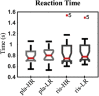
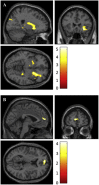
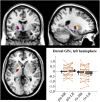
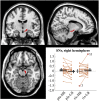
The external part of the globus pallidus (GPe) is a core nucleus of the basal ganglia (BG) whose activity is disrupted under conditions of low dopamine release, as in Parkinson's disease. Current models assume decreased dopamine release in the dorsal striatum results in deactivation of dorsal GPe, which in turn affects motor expression via a regulatory effect on other nuclei of the BG. However, recent studies in healthy and pathological animal models have reported neural dynamics that do not match with this view of the GPe as a relay in the BG circuit. Thus, the computational role of the GPe in the BG is still to be determined. We previously proposed a neural model that revisits the functions of the nuclei of the BG, and this model predicts that GPe encodes values which are amplified under a condition of low striatal dopaminergic drive. To test this prediction, we used an fMRI paradigm involving a within-subject placebo-controlled design, using the dopamine antagonist risperidone, wherein healthy volunteers performed a motor selection and maintenance task under low and high reward conditions. ROI-based fMRI analysis revealed an interaction between reward and dopamine drive manipulations, with increased BOLD activity in GPe in a high compared to low reward condition, and under risperidone compared to placebo. These results confirm the core prediction of our computational model, and provide a new perspective on neural dynamics in the BG and their effects on motor selection and cognitive disorders.
Keywords: Basal ganglia; Dopamine; Globus pallidus; Indirect pathway; Parkinson's disease.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Pallidostriatal Projections Promote β Oscillations in a Dopamine-Depleted Biophysical Network Model.J Neurosci. 2016 May 18;36(20):5556-71. doi: 10.1523/JNEUROSCI.0339-16.2016. J Neurosci. 2016. PMID: 27194335 Free PMC article.
-
Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease.Mov Disord. 2008;23 Suppl 3:S548-59. doi: 10.1002/mds.22062. Mov Disord. 2008. PMID: 18781672 Review.
-
Dopamine depletion leads to pathological synchronization of distinct basal ganglia loops in the beta band.PLoS Comput Biol. 2023 Apr 27;19(4):e1010645. doi: 10.1371/journal.pcbi.1010645. eCollection 2023 Apr. PLoS Comput Biol. 2023. PMID: 37104542 Free PMC article.
-
Roles for globus pallidus externa revealed in a computational model of action selection in the basal ganglia.Neural Netw. 2019 Jan;109:113-136. doi: 10.1016/j.neunet.2018.10.003. Epub 2018 Oct 19. Neural Netw. 2019. PMID: 30414556
-
Synchrony in Parkinson's disease: importance of intrinsic properties of the external globus pallidus.Front Syst Neurosci. 2013 Oct 4;7:60. doi: 10.3389/fnsys.2013.00060. Front Syst Neurosci. 2013. PMID: 24109437 Free PMC article. Review.
Cited by
-
A Multilevel Computational Characterization of Endophenotypes in Addiction.eNeuro. 2018 Jul 17;5(4):ENEURO.0151-18.2018. doi: 10.1523/ENEURO.0151-18.2018. eCollection 2018 Jul-Aug. eNeuro. 2018. PMID: 30073199 Free PMC article.
-
Using pharmacological manipulations to study the role of dopamine in human reward functioning: A review of studies in healthy adults.Neurosci Biobehav Rev. 2021 Jan;120:123-158. doi: 10.1016/j.neubiorev.2020.11.004. Epub 2020 Nov 14. Neurosci Biobehav Rev. 2021. PMID: 33202256 Free PMC article. Review.
-
A change of mind: Globus pallidus activity and effective connectivity during changes in choice selections.Eur J Neurosci. 2021 Apr;53(8):2774-2787. doi: 10.1111/ejn.15142. Epub 2021 Feb 28. Eur J Neurosci. 2021. PMID: 33556221 Free PMC article.
-
Basal ganglia activation localized in MEG using a reward task.Neuroimage Rep. 2021 Jul 28;1(3):100034. doi: 10.1016/j.ynirp.2021.100034. eCollection 2021 Sep. Neuroimage Rep. 2021. PMID: 40567292 Free PMC article.
-
Distinct Roles of the Human Subthalamic Nucleus and Dorsal Pallidum in Parkinson's Disease Impulsivity.Biol Psychiatry. 2022 Feb 15;91(4):370-379. doi: 10.1016/j.biopsych.2021.03.002. Epub 2021 Mar 6. Biol Psychiatry. 2022. PMID: 33993998 Free PMC article.
References
-
- Abdi A., Mallet N., Mohamed F.Y., Sharott A., Dodson P.D., Nakamura K.C., Suri S., Avery S.V., Larvin J.T., Garas F.N., Garas S.N., Vinciati F., Morin S., Bezard E., Baufreton J., Magill P.J. Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J. Neurosci. 2015;35:6667–6688. - PMC - PubMed
-
- Albin R.L., Makowiec R.L., Hollingsworth Z., Sakurai S.Y., Dure L.S.t., 1, Penney J.B., Young A.B. Excitatory amino acidergic pathways and receptors in the basal ganglia. Amino Acids. 1991;1:339–350. - PubMed
-
- Albin R.L., Young A.B., Penney J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

