Mesenchymal Stem Cell Therapy for Ischemic Heart Disease: Systematic Review and Meta-analysis
- PMID: 29482311
- PMCID: PMC5984054
- DOI: 10.15283/ijsc17061
Mesenchymal Stem Cell Therapy for Ischemic Heart Disease: Systematic Review and Meta-analysis
Abstract
Background and objectives: Mesenchymal stem cells (MSC) have emerged as breakthrough treatments for myocardial infarction. However, the efficacy of MSC remains unclear. The aim of the study was to evaluate treatment effect of MSC in terms of mechanical, regenerative, and clinical outcomes for patients with myocardial infarction (MI) using meta-analysis.
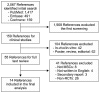
Methods: A systematic search and critical review of MEDLINE, EMBASE, and Cochrane database literature published from inception through December 2017 was performed. The inclusion criteria were randomized controlled trials, studies on patients with myocardial infarction, and studies compared with placebo as a control group.
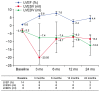
Results: A total of 950 patients from 14 randomized placebo controlled trials were included in the final meta-analysis. MSC treatment showed benefits for mechanical, regenerative, and clinical outcomes. In terms of mechanical outcomes, the LVEF of the MSC treatment group increased by 3.84% (95% CI: 2.32~5.35, I²=43) and the effect was maintained for up to 24 months. Regenerative outcomes were measured by scar mass and WMSI. Scar mass was reduced by -1.13 (95% CI: -1.80 to -0.46, I²=71) and WMSI was reduced by -0.05 (95% CI: -0.07 to -0.03, I²=45) at 6 months after MSC treatment. Mortality rate and incidence of re-hospitalization for HF in MSC group patients trended toward reduced incidence compared to the control group, although this was not statistically significant because of the low event rate.
Conclusions: The findings of this meta-analysis indicate that MSCs can be beneficial in improving heart function in the treatment of MI. However, the efficacy of MSCs must be further explored through large randomized controlled trials based on rigorous research design.
Keywords: Mesenchymal stem cell; Meta-analysis; Myocardial infarction; Systematic review.
Conflict of interest statement
The authors have no conflicting financial interest.
Figures



References
-
- Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. - PubMed
-
- Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. - PubMed
-
- Zhang SN, Sun AJ, Ge JB, Yao K, Huang ZY, Wang KQ, Zou YZ. Intracoronary autologous bone marrow stem cells transfer for patients with acute myocardial infarction: a meta-analysis of randomised controlled trials. Int J Cardiol. 2009;136:178–185. - PubMed
-
- Gyöngyösi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, Marban E, Assmus B, Henry TD, Traverse JH, Moyé LA, Sürder D, Corti R, Huikuri H, Miettinen J, Wöhrle J, Obradovic S, Roncalli J, Malliaras K, Pokushalov E, Romanov A, Kastrup J, Bergmann MW, Atsma DE, Diederichsen A, Edes I, Benedek I, Benedek T, Pejkov H, Nyolczas N, Pavo N, Bergler-Klein J, Pavo IJ, Sylven C, Berti S, Navarese EP, Maurer G ACCRUE Investigators. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015;116:1346–1360. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

