Effectiveness of an educational group intervention in primary healthcare for continued exclusive breast-feeding: PROLACT study
- PMID: 29482516
- PMCID: PMC5828059
- DOI: 10.1186/s12884-018-1679-3
Effectiveness of an educational group intervention in primary healthcare for continued exclusive breast-feeding: PROLACT study
Abstract
Background: The World Health Organization leads a global strategy to promote the initiation and maintenance of breast-feeding. Existing literature shows that education and supportive interventions, both for breast-feeding mothers as well as for healthcare professionals, can increase the proportion of women that use exclusive breast-feeding, however, more evidence is needed on the effectiveness of group interventions.
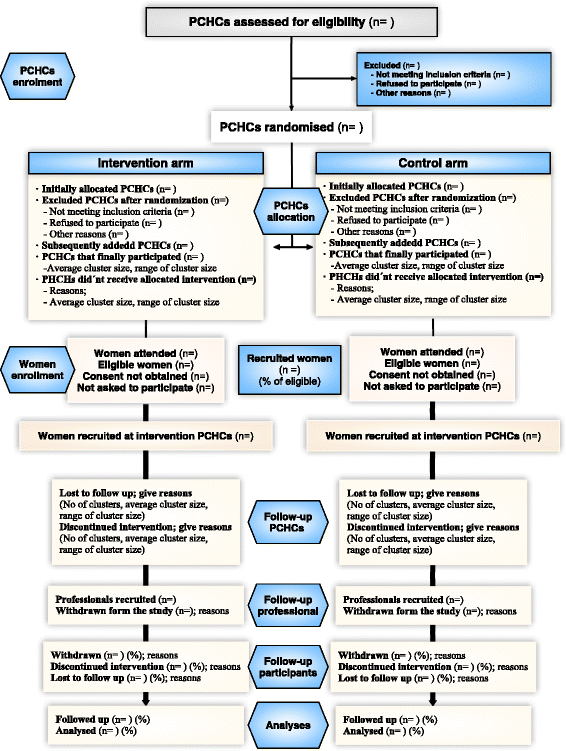
Methods: This study involves a community-based cluster randomised trial conducted at Primary Healthcare Centres in the Community of Madrid (Spain). The project aims to evaluate the effectiveness of an educational group intervention performed by primary healthcare professionals in increasing the proportion of mother-infant pairs using exclusive breastfeeding at six months compared to routine practice. The number of patients required will be 432 (216 in each arm). All mother-infant pairs using exclusive breastfeeding that seek care or information at healthcare centres will be included, as long as the infant is not older than four weeks, and the mother has used exclusive breastfeeding in the last 24 h and who gives consent to participate. The main response variable is mother-infant pairs using exclusive breast-feeding at six months. Main effectiveness will be analysed by comparing the proportion of mother-infant pairs using exclusive breast-feeding at six months between the intervention group and the control group. All statistical tests will be performed with intention-to-treat. The estimation will be adjusted using an explanatory logistic regression model. A survival analysis will be used to compare the two groups using the log-rank test to assess the effect of the intervention on the duration of breastfeeding. The control of potential confounding variables will be performed through the construction of Cox regression models.
Discussion: We must implement strategies with scientific evidence to improve the percentage of exclusive breast-feeding at six months in our environment as established by the WHO. Group education is an instrument used by professionals in Primary Care that favours the acquisition of skills and modification of already-acquired behaviour, all making it a potential method of choice to improve rates of exclusive breast-feeding in this period.
Trial registration: The trial was registered with ClinicalTrials.gov under code number NCT01869920 (Date of registration: June 3, 2013).
Keywords: Breast-feeding; Health education; Primary healthcare.
Conflict of interest statement
Ethics approval and consent to participate
The study has been approved by the Hospital’s Clinical Research Ethics Committee (28 August 2013) and favourably evaluated by the Central Research Commission of the Primary Healthcare Management of Madrid (13 April 2012). The study will be carried out in accordance with the basic principles of the Helsinki Declaration (2013), the Good Clinical Practices (GCP) will be followed along with current Spanish legislation (Real Decreto 223/2004).
Investigator commitment will be requested of all professionals that participate in the study. The investigator will duly inform the subjects that participate in the study and will request their informed consent, signed, and dated in writing. He/she will provide complete and adequate verbal and written information about the nature, purpose and possible risks and benefits of the study.
Any modifications to the protocol which may impact on the conduct of the study, potential benefit of the patient or may affect patient safety, including changes of study objectives, study design, patient population, sample sizes, study procedures, or administrative aspects will require a formal amendment to the protocol. Such amendment will be agreed upon by The Unidad de Apoyo a la Investigación de la Gerencia Asistencial de Atención Primaria de la Comunidad de Madrid.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Proyecto de la UE sobre promoción de la lactancia en Europa. Protección, promoción y apoyo a la lactancia en Europa: plan estratégico para la acción. Comisión Europea, Dirección pública de Salud y Control de Riesgos, Luxemburgo, 2004. Disponible en: http://www.aeped.es/sites/default/files/5-europe_a_blueprint_for_action.pdf
-
- OMS . Indicadores para evaluar las prácticas de alimentación del lactante y del niño pequeño: conclusiones de la reunión de consenso llevada a cabo del 6 al 8 de nov 2007 en Washington, DC. Estados Unidos. 2007.
-
- Oddy WH. The impact of breastmilk on infant and child health. Breastfeed Rev. 2002;10:5–18. - PubMed
-
- American College of Obstetricians and Gynecologists. Breastlfeeding: maternal and infant aspects. ACOG educational. Bulletin Julio. 2000:258.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical