Lessons learned from the blockade of immune checkpoints in cancer immunotherapy
- PMID: 29482595
- PMCID: PMC6389077
- DOI: 10.1186/s13045-018-0578-4
Lessons learned from the blockade of immune checkpoints in cancer immunotherapy
Abstract
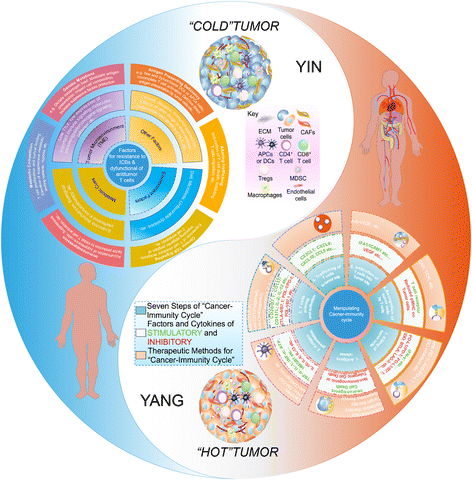
The advent of immunotherapy, especially checkpoint inhibitor-based immunotherapy, has provided novel and powerful weapons against cancer. Because only a subset of cancer patients exhibit durable responses, further exploration of the mechanisms underlying the resistance to immunotherapy in the bulk of cancer patients is merited. Such efforts may help to identify which patients could benefit from immune checkpoint blockade. Given the existence of a great number of pathways by which cancer can escape immune surveillance, and the complexity of tumor-immune system interaction, development of various combination therapies, including those that combine with conventional therapies, would be necessary. In this review, we summarize the current understanding of the mechanisms by which resistance to checkpoint blockade immunotherapy occurs, and outline how actionable combination strategies may be derived to improve clinical outcomes for patients.
Keywords: Cancer immunotherapy; Combination immunotherapy; Immune checkpoint blockade; Resistance mechanism; Tumor microenvironment.
Conflict of interest statement
Ethics approval and consent to participate
This is not applicable for this review.
Consent for publication
This is not applicable for this review.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

