A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults
- PMID: 29482717
- PMCID: PMC5862527
- DOI: 10.7554/eLife.33478
A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults
Erratum in
-
Correction: A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults.Elife. 2023 Mar 21;12:e87888. doi: 10.7554/eLife.87888. Elife. 2023. PMID: 36943904 Free PMC article.
Abstract
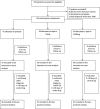
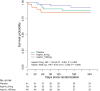
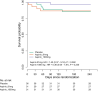
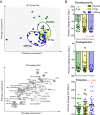
Adjunctive dexamethasone reduces mortality from tuberculous meningitis (TBM) but not disability, which is associated with brain infarction. We hypothesised that aspirin prevents TBM-related brain infarction through its anti-thrombotic, anti-inflammatory, and pro-resolution properties. We conducted a randomised controlled trial in HIV-uninfected adults with TBM of daily aspirin 81 mg or 1000 mg, or placebo, added to the first 60 days of anti-tuberculosis drugs and dexamethasone (NCT02237365). The primary safety endpoint was gastro-intestinal or cerebral bleeding by 60 days; the primary efficacy endpoint was new brain infarction confirmed by magnetic resonance imaging or death by 60 days. Secondary endpoints included 8-month survival and neuro-disability; the number of grade 3 and 4 and serious adverse events; and cerebrospinal fluid (CSF) inflammatory lipid mediator profiles. 41 participants were randomised to placebo, 39 to aspirin 81 mg/day, and 40 to aspirin 1000 mg/day between October 2014 and May 2016. TBM was proven microbiologically in 92/120 (76.7%) and baseline brain imaging revealed ≥1 infarct in 40/114 (35.1%) participants. The primary safety outcome occurred in 5/36 (13.9%) given placebo, and in 8/35 (22.9%) and 8/40 (20.0%) given 81 mg and 1000 mg aspirin, respectively (p=0.59). The primary efficacy outcome occurred in 11/38 (28.9%) given placebo, 8/36 (22.2%) given aspirin 81 mg, and 6/38 (15.8%) given 1000 mg aspirin (p=0.40). Planned subgroup analysis showed a significant interaction between aspirin treatment effect and diagnostic category (Pheterogeneity = 0.01) and suggested a potential reduction in new infarcts and deaths by day 60 in the aspirin treated participants with microbiologically confirmed TBM (11/32 (34.4%) events in placebo vs. 4/27 (14.8%) in aspirin 81 mg vs. 3/28 (10.7%) in aspirin 1000 mg; p=0.06). CSF analysis demonstrated aspirin dose-dependent inhibition of thromboxane A2 and upregulation of pro-resolving CSF protectins. The addition of aspirin to dexamethasone may improve outcomes from TBM and warrants investigation in a large phase 3 trial.
Keywords: Mycobacterium tuberculosis; aspirin; clinical trial; human; infarction; infectious disease; microbiology; tuberculous meningitis.
Plain language summary
The deadliest form of tuberculosis is tuberculosis meningitis (TBM), which causes inflammation in the brain. Even with the best treatment available, about half of patients with TBM become disabled or die, often because they have a stroke. Strokes are caused by blood clots or other blockages in blood vessels in the brain. Aspirin is known to prevent blood clots and helps reduce inflammation. Some scientists wonder if it might help patients with TBM by preventing blockages in blood vessels. Now, Nguyen et al. show that adding aspirin to existing TBM treatments may reduce strokes in some patients. In the experiments, 120 patients with TBM were randomly assigned to receive a low dose of aspirin (81 mg/day), a high dose of aspirin (1000mg/day), or an identical tablet that contained no medication. All the patients also took the anti-tuberculosis drugs and steroids usually used to treat the condition. Both doses of aspirin appeared to be safe. Patients who received aspirin were less likely to have a stroke or die in the first two months of treatment than patients who received the fake pill. But the difference was so small it could have been caused by chance. In the 92 patients with clear evidence of tuberculosis bacteria in their brains, the benefit of aspirin was larger and unlikely to be due to chance. The benefit was greatest for those who received the higher dose of aspirin, only 10.7% of these patients died or had a stroke, compared with 14.8% of those who received a low dose of aspirin, or 34% of those who received the fake pill. Next, Nguyen et al. looked at brain fluid taken from the TBM patients before and after they received the aspirin or fake medication. The experiments showed that patients treated with high dose aspirin had much lower levels of a clot-promoting substance called thromboxane A2 and more anti-inflammatory molecules. Larger studies are needed in children and adults to confirm that aspirin helps prevent strokes or death in patients with TBM. Studies are also needed on patients who have both TBM and HIV infections. But if more studies show aspirin is safe and effective, adding this medication to TBM treatment may be an inexpensive way to prevent death or disability.
© 2018, Mai et al.
Conflict of interest statement
NM, ND, NP, RC, LT, NT, HN, NH, NH, AH, JD, LL, DT, LM, EK, MW, RG, DS, NC, JD, GT No competing interests declared
Figures


Comment in
-
Can aspirin help?Elife. 2018 Mar 21;7:e35906. doi: 10.7554/eLife.35906. Elife. 2018. PMID: 29560861 Free PMC article.
References
-
- Agresti A. Categorical Data Analysis. 2nd edn. Wiley; 2002. - DOI
-
- Ardito F, Posteraro B, Sanguinetti M, Zanetti S, Fadda G. Evaluation of BACTEC Mycobacteria Growth Indicator Tube (MGIT 960) automated system for drug susceptibility testing of Mycobacterium tuberculosis. Journal of Clinical Microbiology. 2001;39:4440–4444. doi: 10.1128/JCM.39.12.4440-4444.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

