Why plants make puzzle cells, and how their shape emerges
- PMID: 29482719
- PMCID: PMC5841943
- DOI: 10.7554/eLife.32794
Why plants make puzzle cells, and how their shape emerges
Abstract
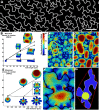
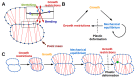
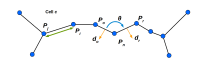
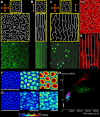
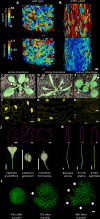
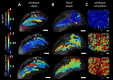
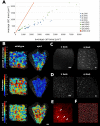
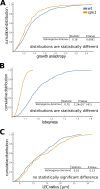
The shape and function of plant cells are often highly interdependent. The puzzle-shaped cells that appear in the epidermis of many plants are a striking example of a complex cell shape, however their functional benefit has remained elusive. We propose that these intricate forms provide an effective strategy to reduce mechanical stress in the cell wall of the epidermis. When tissue-level growth is isotropic, we hypothesize that lobes emerge at the cellular level to prevent formation of large isodiametric cells that would bulge under the stress produced by turgor pressure. Data from various plant organs and species support the relationship between lobes and growth isotropy, which we test with mutants where growth direction is perturbed. Using simulation models we show that a mechanism actively regulating cellular stress plausibly reproduces the development of epidermal cell shape. Together, our results suggest that mechanical stress is a key driver of cell-shape morphogenesis.
Keywords: computational biology; developmental biology; growth; modelling; morphogenesis; organ shape; pavement cells; plant development; stem cells; systems biology.
© 2018, Sapala et al.
Conflict of interest statement
AS, AR, AR, MD, LH, HH, SV, GM, CL, AH, OH, AR, MT, PP, RS No competing interests declared
Figures






References
-
- Barbier de Reuille P, Routier-Kierzkowska A-L, Kierzkowski D, Bassel GW, Schüpbach T, Tauriello G, Bajpai N, Strauss S, Weber A, Kiss A, Burian A, Hofhuis H, Sapala A, Lipowczan M, Heimlicher MB, Robinson S, Bayer EM, Basler K, Koumoutsakos P, Roeder AHK, Aegerter-Wilmsen T, Nakayama N, Tsiantis M, Hay A, Kwiatkowska D, Xenarios I, Kuhlemeier C, Smith RS. MorphoGraphX: A platform for quantifying morphogenesis in 4D. eLife. 2015;4:e05864. doi: 10.7554/eLife.05864. - DOI - PMC - PubMed
-
- Bassel GW, Stamm P, Mosca G, Barbier de Reuille P, Gibbs DJ, Winter R, Janka A, Holdsworth MJ, Smith RS. Mechanical constraints imposed by 3D cellular geometry and arrangement modulate growth patterns in the Arabidopsis embryo. PNAS. 2014;111:8685–8690. doi: 10.1073/pnas.1404616111. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- 615739/ERC_/European Research Council/International
- SystemsX.ch iPhD grant 2010/073/SNSF_/Swiss National Science Foundation/Switzerland
- ERC-2013-CoG-615739 'MechanoDevo'/ERC_/European Research Council/International
- Marie Skłodowska-Curie individual fellowship (Horizon 2020 703886)/MCCC_/Marie Curie/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

