The Pharmacology of CD38/NADase: An Emerging Target in Cancer and Diseases of Aging
- PMID: 29482842
- PMCID: PMC5885288
- DOI: 10.1016/j.tips.2018.02.001
The Pharmacology of CD38/NADase: An Emerging Target in Cancer and Diseases of Aging
Abstract
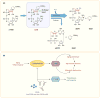
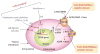
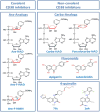
Recent reports indicate that intracellular NAD levels decline in tissues during chronological aging, and that therapies aimed at increasing cellular NAD levels could have beneficial effects in many age-related diseases. The protein CD38 (cluster of differentiation 38) is a multifunctional enzyme that degrades NAD and modulates cellular NAD homeostasis. At the physiological level, CD38 has been implicated in the regulation of metabolism and in the pathogenesis of multiple conditions including aging, obesity, diabetes, heart disease, asthma, and inflammation. Interestingly, many of these functions are mediated by CD38 enzymatic activity. In addition, CD38 has also been identified as a cell-surface marker in hematologic cancers such as multiple myeloma, and a cytotoxic anti-CD38 antibody has been approved by the FDA for use in this disease. Although this is a remarkable development, killing CD38-positive tumor cells with cytotoxic anti-CD38 antibodies is only one of the potential pharmacological uses of targeting CD38. The present review discusses the biology of the CD38 enzyme and the current state of development of pharmacological tools aimed at CD38, and explores how these agents may represent a novel approach for treating human conditions including cancer, metabolic disease, and diseases of aging.
Keywords: CD38; NAD(+); NADase; aging; antibodies; cancer and metabolism; sirtuins; small molecules.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


References
-
- Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

