Radiation Dose-Volume Effects for Liver SBRT
- PMID: 29482870
- PMCID: PMC6095822
- DOI: 10.1016/j.ijrobp.2017.12.290
Radiation Dose-Volume Effects for Liver SBRT
Abstract
Stereotactic body radiation therapy (SBRT) has emerged as an effective, noninvasive treatment option for primary liver cancer and metastatic disease occurring in the liver. Although SBRT can be highly effective for establishing local control in hepatic malignancies, a tradeoff exists between tumor control and normal tissue complications. The objective of the present study was to review the normal tissue dose-volume effects for SBRT-induced liver and gastrointestinal toxicities and derive normal tissue complication probability models.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: none.
Figures
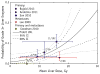
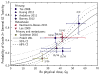
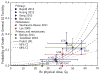
References
-
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int J Cancer. 2010;127:2893–2917. - PubMed
-
- Paley MR, Ros PR. Hepatic metastases. Radiol Clin North Am. 1998;36:349–363. - PubMed
-
- Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. - PubMed
-
- Aloia TA, Vauthey J-N, Loyer EM, et al. Solitary colorectal liver metastasis: Resection determines outcome. Arch Surg. 2006;141:460–467. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

