Otopetrin-1: A sour-tasting proton channel
- PMID: 29483132
- PMCID: PMC5839727
- DOI: 10.1085/jgp.201812003
Otopetrin-1: A sour-tasting proton channel
Erratum in
-
Correction: Otopetrin-1: A sour-tasting proton channel.J Gen Physiol. 2018 Jun 4;150(6):891. doi: 10.1085/jgp.20181200305102018c. Epub 2018 May 16. J Gen Physiol. 2018. PMID: 29769228 Free PMC article. No abstract available.
Abstract
Ramsey and DeSimone highlight the recent discovery of a new family of proton channels.
Figures
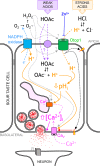
Comment on
-
An evolutionarily conserved gene family encodes proton-selective ion channels.Science. 2018 Mar 2;359(6379):1047-1050. doi: 10.1126/science.aao3264. Epub 2018 Jan 25. Science. 2018. PMID: 29371428 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

