Allele-Specific Mechanisms of Activation of MEK1 Mutants Determine Their Properties
- PMID: 29483135
- PMCID: PMC6112572
- DOI: 10.1158/2159-8290.CD-17-1452
Allele-Specific Mechanisms of Activation of MEK1 Mutants Determine Their Properties
Abstract
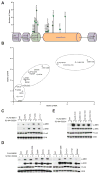
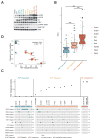
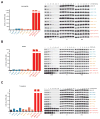
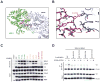
Mutations at multiple sites in MEK1 occur in cancer, suggesting that their mechanisms of activation might be different. We analyzed 17 tumor-associated MEK1 mutants and found that they drove ERK signaling autonomously or in a RAS/RAF-dependent manner. The latter are sensitive to feedback inhibition of RAF, which limits their functional output, and often cooccur with RAS or RAF mutations. They act as amplifiers of RAF signaling. In contrast, another class of mutants deletes a hitherto unrecognized negative regulatory segment of MEK1, is RAF- and phosphorylation-independent, is unaffected by feedback inhibition of upstream signaling, and drives high ERK output and transformation in the absence of RAF activity. Moreover, these RAF-independent mutants are insensitive to allosteric MEK inhibitors, which preferentially bind to the inactivated form of MEK1. All the mutants are sensitive to an ATP-competitive MEK inhibitor. Thus, our study comprises a novel therapeutic strategy for tumors driven by RAF-independent MEK1 mutants.Significance: Mutants with which MEK1 mutants coexist and their sensitivity to inhibitors are determined by allele-specific properties. This study shows the importance of functional characterization of mutant alleles in single oncogenes and identifies a new class of MEK1 mutants, insensitive to current MEK1 inhibitors but treatable with a new ATP-competitive inhibitor. Cancer Discov; 8(5); 648-61. ©2018 AACR.See related commentary by Maust et al., p. 534This article is highlighted in the In This Issue feature, p. 517.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures


Comment in
-
Oncogenic Mutants of MEK1: A Trilogy Unfolds.Cancer Discov. 2018 May;8(5):534-536. doi: 10.1158/2159-8290.CD-18-0192. Cancer Discov. 2018. PMID: 29716939
References
-
- Dong C, Waters SB, Holt KH, Pessin JE. SOS phosphorylation and disassociation of the Grb2-SOS complex by the ERK and JNK signaling pathways. J Biol Chem. 1996;271:6328–32. - PubMed
-
- Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17:215–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

