Peroxisome Proliferator-Activated Receptor γ and Its Role in Adipocyte Homeostasis and Thiazolidinedione-Mediated Insulin Sensitization
- PMID: 29483301
- PMCID: PMC5954194
- DOI: 10.1128/MCB.00677-17
Peroxisome Proliferator-Activated Receptor γ and Its Role in Adipocyte Homeostasis and Thiazolidinedione-Mediated Insulin Sensitization
Abstract
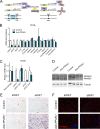
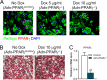
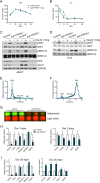
Adipose tissue is a dynamic organ that makes critical contributions to whole-body metabolic homeostasis. Although recent studies have revealed that different fat depots have distinct molecular signatures, metabolic functions and adipogenic mechanisms, peroxisome proliferator-activated receptor γ (PPARγ) is still widely viewed as the master regulator of adipogenesis and critical for maintaining mature adipocyte function. Using an inducible, adipocyte-specific knockout system, we explored the role of PPARγ in mature adipocytes in vivo Short-term PPARγ deficiency in adipocytes reduces whole-body insulin sensitivity, but adipocytes are viable both in vitro and in vivo However, after exposure to a high-fat diet, even short-term PPARγ deficiency leads to rapid adipocyte death. When mature adipocytes are depleted of both PPARγ and CCAAT-enhancer-binding protein α (C/EBPα), they are rapidly depleted of lipids and undergo adipocyte death, both in vitro and in vivo Surprisingly, although thiazolidinediones (TZDs; PPARγ agonists) are thought to act mainly on PPARγ, PPARγ in adipocytes is not required for the whole-body insulin-sensitizing effect of TZDs. This offers new mechanistic aspects of PPARγ/TZD action and its effect on whole-body metabolic homeostasis.
Keywords: C/EBPα; PPARγ; adipocyte; adipose tissue; inducible knockout; insulin sensitization; mouse model; obesity; thiazolidinedione.
Copyright © 2018 American Society for Microbiology.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
