Basic and Clinical Evidence of an Alternative Method to Produce Vivo Nanofat
- PMID: 29483394
- PMCID: PMC5850676
- DOI: 10.4103/0366-6999.226074
Basic and Clinical Evidence of an Alternative Method to Produce Vivo Nanofat
Abstract
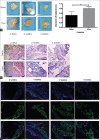
Background: Fat grafting technologies are popularly used in plastic and reconstructive surgery. Due to its size limitation, it is hard to directly inject untreated fat tissue into the dermal layer. Nanofat, which was introduced by Tonnard, solves this problem by mechanically emulsifying fat tissue. However, the viability of the cells was greatly destroyed. In this study, we reported a new method by "gently" digesting the fat tissue to produce viable adipocytes, progenitors, and stromal stem cells using collagenase I digestion and centrifugation. This was named "Vivo nanofat".
Methods: Human liposuction aspirates were obtained from five healthy female donors with mean age of 28.7 ± 5.6 years. Colony-forming assay, flow cytometry analysis, and adipogenic and osteogenic induction of the adherent cells from the Vivo nanofat were used to characterize the adipose mesenchymal stem cells (MSCs). To investigate in vivo survival, we respectively injected Vivo nanofat and nanofat subcutaneously to the back of 8-week-old male BALB/c nude mice. Samples were harvested 2 days, 2 weeks, and 4 weeks postinjection for measurement, hematoxylin and eosin staining, and immunostaining.
Results: Our results showed that the Vivo nanofat contained a large number of colony-forming cells. These cells expressed MSC markers and had multi-differentiative potential. In vivo transplantation showed that the Vivo nanofat had lower resorption ratio than that of nanofat. The size of the transplanted nanofat was obviously smaller than that of Vivo nanofat 4 weeks postinjection (0.50 ± 0.17 cm vs. 0.81 ± 0.07 cm, t = -5783, P = 0.01).
Conclusion: Vivo nanofat may serve as a cell fraction injectable through a fine needle; this could be used for cosmetic applications.
自体纳米活性脂肪细胞制备及基础研究和临床观察摘要背景:目前,脂肪填充技术已经广泛应用于整形和美容医学。然而,直接吸脂获得的脂肪组织颗粒较大,无法直接进行皮内注射。Tonnard报道的纳米脂肪通过物理方法从而获得了小颗粒的乳化脂肪。但是,该方法在处理脂肪过程中,脂肪细胞的活性被严重的破坏。 方法:本研究中,我们报道了一种新的先通过I型胶原酶化学消化,再离心获得小颗粒脂肪的方法。获得的脂肪组织包括脂肪细胞、脂肪祖细胞及间充质干细胞。我们将之命名为“纳米活性脂肪”。我们通过细胞集落形成实验、流式细胞术、成脂和成骨诱导,确定了获得的纳米活性脂肪中含有脂肪间充质干细胞活性成分。我们通过在裸鼠背部皮下注射纳米活性脂肪和纳米脂肪进行对比,研究了纳米脂肪在体移植后的存活能力。 结果:我们的结果显示,和传统的纳米脂肪相比,纳米活性脂肪中含有更多的干细胞活性成分。这些细胞表达间充质细胞标记物并且具有多向分化潜能。在体实验表明,纳米活性脂肪成活率更高,吸收率更低。在移植后第四周取材,纳米活性脂肪体积大于传统的纳米脂肪 (0.50 ± 0.17 cm vs. 0.81 ± 0.07 cm)。 结论:我们报道的纳米活性脂肪具有可精细注射、活性细胞成分多的特点,在整形和美容医学有较强的应用前景。.
Keywords: Adipose Tissue; Cell Therapy; Mesenchymal Stromal Cells; Rejuvenation.
Conflict of interest statement
There are no conflicts of interest.
Figures



References
-
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. - PubMed
-
- Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ, et al. Fat grafting: Evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130:249–58. doi: 10.1097/PRS.0b013e318254b4d3. - PubMed
-
- Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno S, et al. The fate of adipocytes after nonvascularized fat grafting: Evidence of early death and replacement of adipocytes. Plast Reconstr Surg. 2012;129:1081–92. doi: 10.1097/PRS.0b013e31824a2b19. - PubMed
-
- Kato H, Mineda K, Eto H, Doi K, Kuno S, Kinoshita K, et al. Degeneration, regeneration, and cicatrization after fat grafting: Dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg. 2014;133:303e–13e. doi: 10.1097/PRS.0000000000000066. - PubMed
-
- Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H, et al. Nanofat grafting: Basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017–26. doi: 10.1097/PRS.0000000000000333. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

