The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens
- PMID: 29483501
- PMCID: PMC5826932
- DOI: 10.1038/s41467-018-03236-6
The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens
Abstract
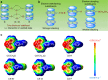
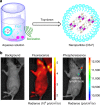
Organic luminogens with persistent room temperature phosphorescence (RTP) have attracted great attention for their wide applications in optoelectronic devices and bioimaging. However, these materials are still very scarce, partially due to the unclear mechanism and lack of designing guidelines. Herein we develop seven 10-phenyl-10H-phenothiazine-5,5-dioxide-based derivatives, reveal their different RTP properties and underlying mechanism, and exploit their potential imaging applications. Coupled with the preliminary theoretical calculations, it is found that strong π-π interactions in solid state can promote the persistent RTP. Particularly, CS-CF3 shows the unique photo-induced phosphorescence in response to the changes in molecular packing, further confirming the key influence of the molecular packing on the RTP property. Furthermore, CS-F with its long RTP lifetime could be utilized for real-time excitation-free phosphorescent imaging in living mice. Thus, our study paves the way for the development of persistent RTP materials, in both the practical applications and the inherent mechanism.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

