MicroRNA Contents in Matrix Vesicles Produced by Growth Plate Chondrocytes are Cell Maturation Dependent
- PMID: 29483516
- PMCID: PMC5826934
- DOI: 10.1038/s41598-018-21517-4
MicroRNA Contents in Matrix Vesicles Produced by Growth Plate Chondrocytes are Cell Maturation Dependent
Abstract
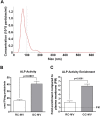
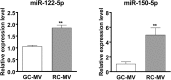
Chondrocytes at different maturation states in the growth plate produce matrix vesicles (MVs), membrane organelles found in the extracellular matrix, with a wide range of contents, such as matrix processing enzymes and receptors for hormones. We have shown that MVs harvested from growth zone (GC) chondrocyte cultures contain abundant small RNAs, including miRNAs. Here, we determined whether RNA also exists in MVs produced by less mature resting zone (RC) chondrocytes and, if so, whether it differs from the RNA in MVs produced by GC cells. Our results showed that RNA, small RNA specifically, was present in RC-MVs, and it was well-protected from RNase by the phospholipid membrane. A group of miRNAs was enriched in RC-MVs compared RC-cells, suggesting that miRNAs are selectively packaged into MVs. High throughput array and RNA sequencing showed that ~39% miRNAs were differentially expressed between RC-MVs and GC-MVs. Individual RT-qPCR also confirmed that miR-122-5p and miR-150-5p were expressed at significantly higher levels in RC-MVs compared to GC-MVs. This study showed that growth plate chondrocytes at different differentiation stages produce different MVs with different miRNA contents, further supporting extracellular vesicle miRNAs play a role as "matrisomes" that mediate the cell-cell communication in cartilage and bone development.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

