Characterization of the quinol-dependent nitric oxide reductase from the pathogen Neisseria meningitidis, an electrogenic enzyme
- PMID: 29483528
- PMCID: PMC5826923
- DOI: 10.1038/s41598-018-21804-0
Characterization of the quinol-dependent nitric oxide reductase from the pathogen Neisseria meningitidis, an electrogenic enzyme
Abstract
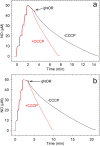
Bacterial nitric oxide reductases (NORs) catalyse the reduction of NO to N2O and H2O. NORs are found either in denitrification chains, or in pathogens where their primary role is detoxification of NO produced by the immune defense of the host. Although NORs belong to the heme-copper oxidase superfamily, comprising proton-pumping O2-reducing enzymes, the best studied NORs, cNORs (cytochrome c-dependent), are non-electrogenic. Here, we focus on another type of NOR, qNOR (quinol-dependent). Recombinant qNOR from Neisseria meningitidis, a human pathogen, purified from Escherichia coli, showed high catalytic activity and spectroscopic properties largely similar to cNORs. However, in contrast to cNOR, liposome-reconstituted qNOR showed respiratory control ratios above two, indicating that NO reduction by qNOR was electrogenic. Further, we determined a 4.5 Å crystal structure of the N. meningitidis qNOR, allowing exploration of a potential proton transfer pathway from the cytoplasm by mutagenesis. Most mutations had little effect on the activity, however the E-498 variants were largely inactive, while the corresponding substitution in cNOR was previously shown not to induce significant effects. We thus suggest that, contrary to cNOR, the N. meningitidis qNOR uses cytoplasmic protons for NO reduction. Our results allow possible routes for protons to be discussed.
Conflict of interest statement
The authors declare no competing interests.
Figures




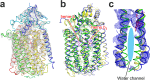

References
-
- Bell LC, Richardson DJ, Ferguson SJ. Identification of nitric oxide reductase activity in Rhodobacter capsulatus: the electron transport pathway can either use or bypass both cytochrome c2 and the cytochrome bc1 complex. J. Gen. Microbiol. 1992;138:437–443. doi: 10.1099/00221287-138-3-437. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

