Sculpting nanoparticle dynamics for single-bacteria-level screening and direct binding-efficiency measurement
- PMID: 29483548
- PMCID: PMC5827716
- DOI: 10.1038/s41467-018-03156-5
Sculpting nanoparticle dynamics for single-bacteria-level screening and direct binding-efficiency measurement
Erratum in
-
Author Correction: Sculpting nanoparticle dynamics for single-bacteria-level screening and direct binding-efficiency measurement.Nat Commun. 2019 Mar 12;10(1):1227. doi: 10.1038/s41467-019-09171-4. Nat Commun. 2019. PMID: 30862795 Free PMC article.
Abstract
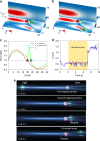
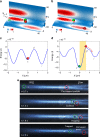
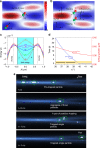
Particle trapping and binding in optical potential wells provide a versatile platform for various biomedical applications. However, implementation systems to study multi-particle contact interactions in an optical lattice remain rare. By configuring an optofluidic lattice, we demonstrate the precise control of particle interactions and functions such as controlling aggregation and multi-hopping. The mean residence time of a single particle is found considerably reduced from 7 s, as predicted by Kramer's theory, to 0.6 s, owing to the mechanical interactions among aggregated particles. The optofluidic lattice also enables single-bacteria-level screening of biological binding agents such as antibodies through particle-enabled bacteria hopping. The binding efficiency of antibodies could be determined directly, selectively, quantitatively and efficiently. This work enriches the fundamental mechanisms of particle kinetics and offers new possibilities for probing and utilising unprecedented biomolecule interactions at single-bacteria level.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

