The interdependence of excitation and inhibition for the control of dynamic breathing rhythms
- PMID: 29483589
- PMCID: PMC5827754
- DOI: 10.1038/s41467-018-03223-x
The interdependence of excitation and inhibition for the control of dynamic breathing rhythms
Abstract
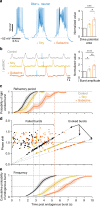
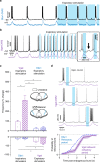
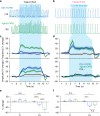
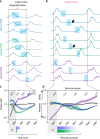
The preBötzinger Complex (preBötC), a medullary network critical for breathing, relies on excitatory interneurons to generate the inspiratory rhythm. Yet, half of preBötC neurons are inhibitory, and the role of inhibition in rhythmogenesis remains controversial. Using optogenetics and electrophysiology in vitro and in vivo, we demonstrate that the intrinsic excitability of excitatory neurons is reduced following large depolarizing inspiratory bursts. This refractory period limits the preBötC to very slow breathing frequencies. Inhibition integrated within the network is required to prevent overexcitation of preBötC neurons, thereby regulating the refractory period and allowing rapid breathing. In vivo, sensory feedback inhibition also regulates the refractory period, and in slowly breathing mice with sensory feedback removed, activity of inhibitory, but not excitatory, neurons restores breathing to physiological frequencies. We conclude that excitation and inhibition are interdependent for the breathing rhythm, because inhibition permits physiological preBötC bursting by controlling refractory properties of excitatory neurons.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






References
-
- Yokosawa K, Kimura K, Chitose R, Momiki T, Kuriki S. Alpha-band rhythm suppression during memory recall reflecting memory performance. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016;2016:4539–4542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

