LINC01410-miR-532-NCF2-NF-kB feedback loop promotes gastric cancer angiogenesis and metastasis
- PMID: 29483646
- PMCID: PMC5955863
- DOI: 10.1038/s41388-018-0162-y
LINC01410-miR-532-NCF2-NF-kB feedback loop promotes gastric cancer angiogenesis and metastasis
Erratum in
-
Correction: LINC01410-miR-532-NCF2- NF-kB feedback loop promotes gastric cancer angiogenesis and metastasis.Oncogene. 2021 Aug;40(33):5247-5252. doi: 10.1038/s41388-021-01796-4. Oncogene. 2021. PMID: 34183773 Free PMC article. No abstract available.
Retraction in
-
Retraction Note: LINC01410-miR-532-NCF2-NF-kB feedback loop promotes gastric cancer angiogenesis and metastasis.Oncogene. 2023 Mar;42(14):1158. doi: 10.1038/s41388-023-02635-4. Oncogene. 2023. PMID: 36879118 Free PMC article. No abstract available.
Abstract
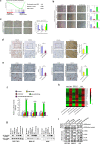
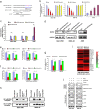
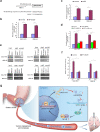
Dysregulation of non-coding RNAs, including miRNAs and lncRNAs has been reported to play vital roles in gastric cancer (GC) carcinogenesis, but the mechanism involved is largely unknown. Using the cancer genome atlas (TCGA) data set and bioinformatics analyses, we identified miR-532-5p as a potential tumor suppressor in GC, and found that lncRNA LINC01410 might be a negative regulator of miR-532-5p. We then conducted a series of in vivo and in vitro assays to explore the effect of LINC01410 on miR-532-5p-mediated GC malignancy and the underlying mechanism involved. MiR-532-5p overexpression inhibited GC metastasis and angiogenesis in vitro and in vivo, whereas miR-532-5p silencing had the opposite effect. Further study showed that miR-532-5p attenuated NF-κB signaling by directly inhibiting NCF2 expression, while miR-532-5p silencing in GC enhanced NF-κB activity. Furthermore, we demonstrated miR-532-5p down-regulation was caused by aberrantly high expression of LINC01410 in GC. Mechanistically, overexpression of LINC01410 promoted GC angiogenesis and metastasis by binding to and suppressing miR-532-5p, which resulted in up-regulation of NCF2 and sustained NF-κB pathway activation. Interestingly, NCF2 could in turn increase the promoter activity and expression of LINC01410 via NF-κB, thus forming a positive feedback loop that drives the malignant behavior of GC. Finally, high expression of LINC01410, along with low expression of miR-532-5p, was associated with poor survival outcome in GC patients. Our studies uncover a mechanism for constitutive LINC1410-miR-532-5p-NCF2-NF-κB feedback loop activation in GC, and consequently, as a potential therapeutic target in GC treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

