An "off-the-shelf" fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies
- PMID: 29483708
- PMCID: PMC6102094
- DOI: 10.1038/s41375-018-0065-5
An "off-the-shelf" fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies
Abstract
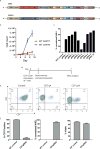
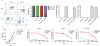
T cell malignancies represent a group of hematologic cancers with high rates of relapse and mortality in patients for whom no effective targeted therapies exist. The shared expression of target antigens between chimeric antigen receptor (CAR) T cells and malignant T cells has limited the development of CAR-T because of unintended CAR-T fratricide and an inability to harvest sufficient autologous T cells. Here, we describe a fratricide-resistant "off-the-shelf" CAR-T (or UCART7) that targets CD7+ T cell malignancies and, through CRISPR/Cas9 gene editing, lacks both CD7 and T cell receptor alpha chain (TRAC) expression. UCART7 demonstrates efficacy against human T cell acute lymphoblastic leukemia (T-ALL) cell lines and primary T-ALL in vitro and in vivo without the induction of xenogeneic GvHD. Fratricide-resistant, allo-tolerant "off-the-shelf" CAR-T represents a strategy for treatment of relapsed and refractory T-ALL and non-Hodgkin's T cell lymphoma without a requirement for autologous T cells.
Conflict of interest statement
Figures




References
-
- Karrman K, Johansson B. Pediatric T-cell acute lymphoblastic leukemia. Genes, Chromosomes and Cancer. 2017;56(2):89–116. - PubMed
-
- Gökbuget N, Arnold R, Böhme A, Fietkau R, Freund M, Ganser A, et al. Acute Leukemias. Springer Berlin Heidelberg; Berlin, Heidelberg: 2008. Treatment of Adult ALL According to Protocols of the German Multicenter Study Group for Adult ALL (GMALL) pp. 167–176.
-
- Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, et al. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003 Oct 01;21(19):3616–3622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

