Exosomes in Pathogen Infections: A Bridge to Deliver Molecules and Link Functions
- PMID: 29483904
- PMCID: PMC5816030
- DOI: 10.3389/fimmu.2018.00090
Exosomes in Pathogen Infections: A Bridge to Deliver Molecules and Link Functions
Abstract
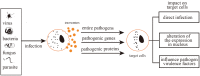
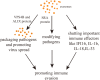
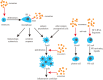
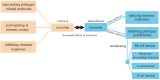
Exosomes are extracellular vesicles derived from cell endocytosis which act as transmitters between cells. They are composed of proteins, lipids, and RNAs through which they participate in cellular crosstalk. Consequently, they play an important role in health and disease. Our view is that exosomes exert a bidirectional regulatory effect on pathogen infections by delivering their content. First, exosomes containing proteins and RNAs derived from pathogens can promote infections in three ways: (1) mediating further infection by transmitting pathogen-related molecules; (2) participating in the immune escape of pathogens; and (3) inhibiting immune responses by favoring immune cell apoptosis. Second, exosomes play anti-infection roles through: (1) inhibiting pathogen proliferation and infection directly; (2) inducing immune responses such as those related to the function of monocyte-macrophages, NK cells, T cells, and B cells. We believe that exosomes act as "bridges" during pathogen infections through the mechanisms mentioned above. The purpose of this review is to describe present findings regarding exosomes and pathogen infections, and highlight their enormous potential in clinical diagnosis and treatment. We discuss two opposite aspects: infection and anti-infection, and we hypothesize a balance between them. At the same time, we elaborate on the role of exosomes in immune regulation.
Keywords: apoptosis; exosome; immune regulation; infection; pathogen; transmit carrier.
Figures
Similar articles
-
Functions of Cancer-Derived Extracellular Vesicles in Immunosuppression.Arch Immunol Ther Exp (Warsz). 2017 Aug;65(4):311-323. doi: 10.1007/s00005-016-0453-3. Epub 2017 Jan 18. Arch Immunol Ther Exp (Warsz). 2017. PMID: 28101591 Free PMC article. Review.
-
Extracellular Vesicles Deliver Host and Virus RNA and Regulate Innate Immune Response.Int J Mol Sci. 2017 Mar 20;18(3):666. doi: 10.3390/ijms18030666. Int J Mol Sci. 2017. PMID: 28335522 Free PMC article. Review.
-
Functions of Exosomes and Microbial Extracellular Vesicles in Allergy and Contact and Delayed-Type Hypersensitivity.Int Arch Allergy Immunol. 2016;171(1):1-26. doi: 10.1159/000449249. Epub 2016 Nov 8. Int Arch Allergy Immunol. 2016. PMID: 27820941 Free PMC article. Review.
-
Intravacuolar Pathogens Hijack Host Extracellular Vesicle Biogenesis to Secrete Virulence Factors.Front Immunol. 2021 Apr 20;12:662944. doi: 10.3389/fimmu.2021.662944. eCollection 2021. Front Immunol. 2021. PMID: 33959131 Free PMC article. Review.
-
Modulation of Host-Pathogen Communication by Extracellular Vesicles (EVs) of the Protozoan Parasite Leishmania.Front Cell Infect Microbiol. 2019 Apr 11;9:100. doi: 10.3389/fcimb.2019.00100. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31032233 Free PMC article. Review.
Cited by
-
Mechanistic and Therapeutic Implications of Extracellular Vesicles as a Potential Link Between Covid-19 and Cardiovascular Disease Manifestations.Front Cell Dev Biol. 2021 Feb 11;9:640723. doi: 10.3389/fcell.2021.640723. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33644077 Free PMC article.
-
Inhibitors of Bacterial Extracellular Vesicles.Front Microbiol. 2022 Feb 23;13:835058. doi: 10.3389/fmicb.2022.835058. eCollection 2022. Front Microbiol. 2022. PMID: 35283837 Free PMC article. Review.
-
ExoPD-L1: an assistant for tumor progression and potential diagnostic marker.Front Oncol. 2023 Sep 5;13:1194180. doi: 10.3389/fonc.2023.1194180. eCollection 2023. Front Oncol. 2023. PMID: 37736550 Free PMC article. Review.
-
Extracellular RNAs in Bacterial Infections: From Emerging Key Players on Host-Pathogen Interactions to Exploitable Biomarkers and Therapeutic Targets.Int J Mol Sci. 2020 Dec 17;21(24):9634. doi: 10.3390/ijms21249634. Int J Mol Sci. 2020. PMID: 33348812 Free PMC article. Review.
-
Exosomes from Ureaplasma parvum-infected ectocervical epithelial cells promote feto-maternal interface inflammation but are insufficient to cause preterm delivery.Front Cell Dev Biol. 2022 Aug 15;10:931609. doi: 10.3389/fcell.2022.931609. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36046342 Free PMC article.
References
-
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem (1987) 262(19):9412–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

