A New Classification System for IgG4 Autoantibodies
- PMID: 29483905
- PMCID: PMC5816565
- DOI: 10.3389/fimmu.2018.00097
A New Classification System for IgG4 Autoantibodies
Abstract
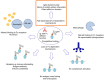
IgG4 autoimmune diseases are characterized by the presence of antigen-specific autoantibodies of the IgG4 subclass and contain well-characterized diseases such as muscle-specific kinase myasthenia gravis, pemphigus, and thrombotic thrombocytopenic purpura. In recent years, several new diseases were identified, and by now 14 antigens targeted by IgG4 autoantibodies have been described. The IgG4 subclass is considered immunologically inert and functionally monovalent due to structural differences compared to other IgG subclasses. IgG4 usually arises after chronic exposure to antigen and competes with other antibody species, thus "blocking" their pathogenic effector mechanisms. Accordingly, in the context of IgG4 autoimmunity, the pathogenicity of IgG4 is associated with blocking of enzymatic activity or protein-protein interactions of the target antigen. Pathogenicity of IgG4 autoantibodies has not yet been systematically analyzed in IgG4 autoimmune diseases. Here, we establish a modified classification system based on Witebsky's postulates to determine IgG4 pathogenicity in IgG4 autoimmune diseases, review characteristics and pathogenic mechanisms of IgG4 in these disorders, and also investigate the contribution of other antibody entities to pathophysiology by additional mechanisms. As a result, three classes of IgG4 autoimmune diseases emerge: class I where IgG4 pathogenicity is validated by the use of subclass-specific autoantibodies in animal models and/or in vitro models of pathogenicity; class II where IgG4 pathogenicity is highly suspected but lack validation by the use of subclass specific antibodies in in vitro models of pathogenicity or animal models; and class III with insufficient data or a pathogenic mechanism associated with multivalent antigen binding. Five out of the 14 IgG4 antigens were validated as class I, five as class II, and four as class III. Antibodies of other IgG subclasses or immunoglobulin classes were present in several diseases and could contribute additional pathogenic mechanisms.
Keywords: IgG4; IgG4 autoimmunity; IgG4-related disease; autoimmunity; muscle-specific kinase myasthenia gravis; neuronal autoantibodies; pemphigus; thrombotic thrombocytopenic purpura.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

