Cow's Milk and Immune Function in the Respiratory Tract: Potential Mechanisms
- PMID: 29483908
- PMCID: PMC5816034
- DOI: 10.3389/fimmu.2018.00143
Cow's Milk and Immune Function in the Respiratory Tract: Potential Mechanisms
Abstract
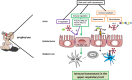
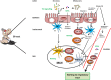
During the last decades, the world has witnessed a dramatic increase in allergy prevalence. Epidemiological evidence shows that growing up on a farm is a protective factor, which is partly explained by the consumption of raw cow's milk. Indeed, recent studies show inverse associations between raw cow's milk consumption in early life and asthma, hay fever, and rhinitis. A similar association of raw cow's milk consumption with respiratory tract infections is recently found. In line with these findings, controlled studies in infants with milk components such as lactoferrin, milk fat globule membrane, and colostrum IgG have shown to reduce respiratory infections. However, for ethical reasons, it is not possible to conduct controlled studies with raw cow's milk in infants, so formal proof is lacking to date. Because viral respiratory tract infections and aeroallergen exposure in children may be causally linked to the development of asthma, it is of interest to investigate whether cow's milk components can modulate human immune function in the respiratory tract and via which mechanisms. Inhaled allergens and viruses trigger local immune responses in the upper airways in both nasal and oral lymphoid tissue. The components present in raw cow's milk are able to promote a local microenvironment in which mucosal immune responses are modified and the epithelial barrier is enforced. In addition, such responses may also be triggered in the gut after exposure to allergens and viruses in the nasal cavity that become available in the GI tract after swallowing. However, these immune cells that come into contact with cow's milk components in the gut must recirculate into the blood and home to the (upper and lower) respiratory tract to regulate immune responses locally. Expression of the tissue homing-associated markers α4β7 and CCR9 or CCR10 on lymphocytes can be influenced by vitamin A and vitamin D3, respectively. Since both vitamins are present in milk, we speculate that raw milk may influence homing of lymphocytes to the upper respiratory tract. This review focuses on potential mechanisms via which cow's milk or its components can influence immune function in the intestine and the upper respiratory tract. Unraveling these complex mechanisms may contribute to the development of novel dietary approaches in allergy and asthma prevention.
Keywords: allergies; asthma; barrier functioning; gut–lung axis; immune regulation; raw cow’s milk; respiratory syncytial virus; upper airways.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

