Agonistic Autoantibodies to the β2-Adrenergic Receptor Involved in the Pathogenesis of Open-Angle Glaucoma
- PMID: 29483909
- PMCID: PMC5816038
- DOI: 10.3389/fimmu.2018.00145
Agonistic Autoantibodies to the β2-Adrenergic Receptor Involved in the Pathogenesis of Open-Angle Glaucoma
Abstract
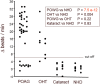
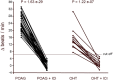
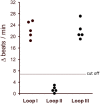
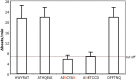
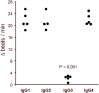
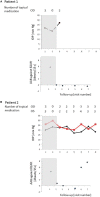
Glaucoma is a frequent ocular disease that may lead to blindness. Primary open-angle glaucoma (POAG) and ocular hypertension (OHT) are common diseases with increased intraocular pressure (IOP), which are mainly responsible for these disorders. Their pathogenesis is widely unknown. We screened the sera of patients with POAG and OHT for the prevalence of autoantibodies (AAb) against G protein-coupled receptors (GPCRs) in comparison to controls. Employing frequency modulation of spontaneously contracting neonatal rat cardiomyocytes in vitro, agonistic GPCR AAb were to be detected in roughly 75% of the patients with POAG and OHT, however, not in controls. Using inhibitory peptides the AAb' target was identified as β2 adrenergic receptor (β2AR). The AAb interact with the second extracellular loop of β2AR. The peptides 181-187 and 186-192 were identified as binding sites of the AAb within the extracellular loop II. The binding of the AAb to β2ARs was verified by surface-plasmon-resonance analysis. The isotype of the AAb was (immunoglobulin) IgG3. In an additional pilot principal-of-proof study, including four patients with POAG, the removal of the AAb against the β2AR and other immunoglobulins G by immunoadsorption resulted in a transient reduction of IOP. These findings might indicate a possible role of agonistic AAb directed against β2ARs in the dynamics of aqueous humor and might support a contribution of adaptive autoimmunity in the etiopathogenesis of POAG and OHT.
Trial registration: ClinicalTrials.gov NCT00494923.
Keywords: agonistic; autoantibodies; glaucoma; immunoadsorption; ocular hypertension; β2-adrenergic receptor.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

