Local cortisol activation is involved in EGF-induced immunosuppression
- PMID: 29484105
- PMCID: PMC5821158
- DOI: 10.1080/19381980.2017.1412018
Local cortisol activation is involved in EGF-induced immunosuppression
Abstract
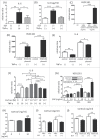
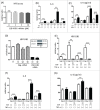
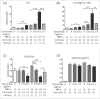
The major effects of the epidermal growth factor receptor (EGFR) signalling pathway on keratinocytes are cell proliferation, cell differentiation, and wound healing. In addition to these effects, an immunosuppressive effect of EGFR signalling has been reported. However, the precise mechanism of immunosuppression by EGFR signalling is not well understood. In this study, we clarified the involvement of increased local cortisol activation in EGFR signalling-induced immunosuppression in keratinocytes. EGF treatment up-regulated the expression of 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) and supernatant cortisol levels in a dose-dependent manner in keratinocytes. 11β-HSD1 is an enzyme that catalyses the conversion of cellular hormonally inactive cortisone into active cortisol. qRT-PCR and ELISA assays indicated that EGF significantly decreased tumour necrosis factor α (TNF- α)-induced interleukin-6 (IL-6) expression in keratinocytes. Similarly, 11β-HSD1 overexpression significantly decreased TNF-α-induced IL-6 expression. We evaluated the role of 11β-HSD1 in immunosuppression through EGFR signalling. Blockade of 11β-HSD1 via 11β-HSD1 inhibitor reversed both the expression and production of TNF-α-induced IL-6, which was decreased by EGF in keratinocytes. Therefore, increased local cortisol activation by 11β-HSD1 is involved in EGFR signalling-induced immunosuppression in keratinocytes. Finally, we evaluated whether EGFR inhibition by cetuximab affects the expression of 11β-HSD1. We found that 0.1 µg cetuximab decreased 11β-HSD1 transcript levels in keratinocytes. The changes in 11β-HSD1 were more apparent in TNF-α-treated cells. As 11β-HSD1 expression in keratinocytes is associated with inflammation and cell proliferation, this mechanism may be associated with adverse skin reactions observed in patients treated with EGFR inhibitors.
Keywords: 11β-hydroxysteroid dehydrogenase; atopic dermatitis; cortisol; epidermal growth factor receptor; inflammation; keratinocyte;.
Figures
Similar articles
-
11β-Hydroxysteroid dehydrogenase 1 contributes to the pro-inflammatory response of keratinocytes.Biochem Biophys Res Commun. 2013 Oct 18;440(2):265-70. doi: 10.1016/j.bbrc.2013.09.065. Epub 2013 Sep 19. Biochem Biophys Res Commun. 2013. PMID: 24055708
-
Local Glucocorticoid Activation by 11β-Hydroxysteroid Dehydrogenase 1 in Keratinocytes: The Role in Hapten-Induced Dermatitis.Am J Pathol. 2016 Jun;186(6):1499-510. doi: 10.1016/j.ajpath.2016.01.014. Epub 2016 Apr 9. Am J Pathol. 2016. PMID: 27070821
-
Activation of Local 11β-Hydroxysteroid Dehydrogenase Type 1 by Diosmetin Enhances Endogenous Glucocorticoid Levels to Alleviate Skin Inflammation: Insights Into a Novel Therapeutic Strategy for Atopic Dermatitis.Exp Dermatol. 2025 Feb;34(2):e70039. doi: 10.1111/exd.70039. Exp Dermatol. 2025. PMID: 39887444
-
Local cortisol/corticosterone activation in skin physiology and pathology.J Dermatol Sci. 2016 Oct;84(1):11-16. doi: 10.1016/j.jdermsci.2016.06.014. Epub 2016 Jun 29. J Dermatol Sci. 2016. PMID: 27431412 Review.
-
Growth hormone, insulin-like growth factor-I and the cortisol-cortisone shuttle.Horm Res. 2001;56 Suppl 1:1-6. doi: 10.1159/000048126. Horm Res. 2001. PMID: 11786677 Review.
Cited by
-
Methotrexate and Cetuximab-Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments.Medicina (Kaunas). 2022 Jan 22;58(2):167. doi: 10.3390/medicina58020167. Medicina (Kaunas). 2022. PMID: 35208492 Free PMC article.
-
Comparative Analysis of Chemical Profiles and Biological Activities of Essential Oils Derived from Torreya grandis Arils and Leaves: In Vitro and In Silico Studies.Plants (Basel). 2024 Sep 21;13(18):2640. doi: 10.3390/plants13182640. Plants (Basel). 2024. PMID: 39339615 Free PMC article.
-
Regional and time course differences in sweat cortisol, glucose, and select cytokine concentrations during exercise.Eur J Appl Physiol. 2023 Aug;123(8):1727-1738. doi: 10.1007/s00421-023-05187-3. Epub 2023 Apr 2. Eur J Appl Physiol. 2023. PMID: 37005963 Free PMC article.
References
-
- Poumay Y, de Rouvroit CL. HB-EGF, the growth factor that accelerates keratinocyte migration, but slows proliferation. J Invest Dermatol 2012;132:2129–30. - PubMed
-
- Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol 2008;128:1365–74. - PubMed
-
- Gao HB, Ge RS, Lakshmi V, Marandici A, Hardy MP. Hormonal regulation of oxidative and reductive activities of 11 beta-hydroxysteroid dehydrogenase in rat Leydig cells. Endocrinology 1997;138:156–61. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
