Insulin receptor sensitization restores neocortical excitation/inhibition balance in a mouse model of autism
- PMID: 29484150
- PMCID: PMC5824550
- DOI: 10.1186/s13229-018-0196-6
Insulin receptor sensitization restores neocortical excitation/inhibition balance in a mouse model of autism
Abstract
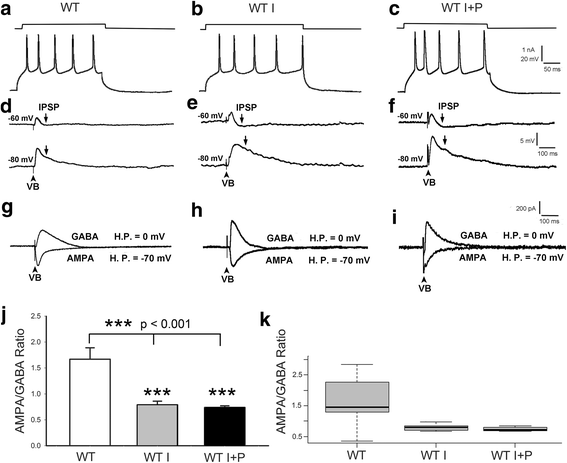
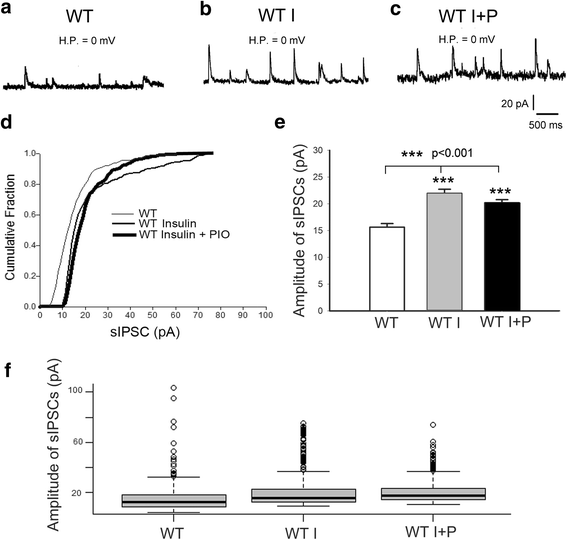
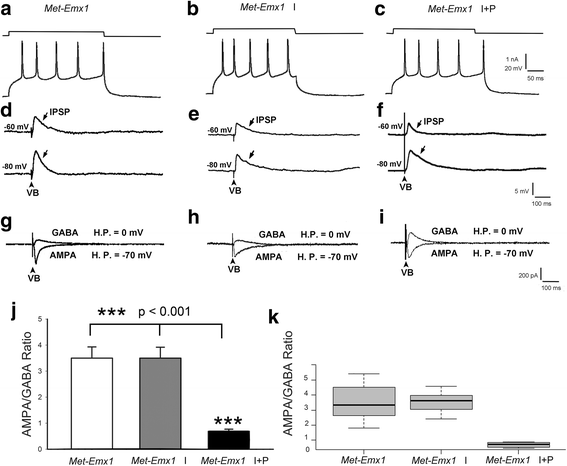
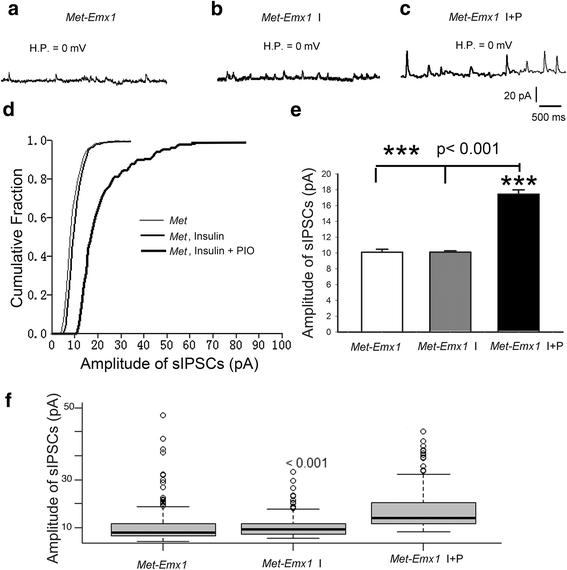
Background: Met receptor tyrosine kinase regulates neurogenesis, differentiation, migration, connectivity, and synaptic plasticity. The human Met gene has been identified as a prominent risk factor for autism spectrum disorder (ASD). Met gene-altered mice serve as useful models for mechanistic studies of ASD. Inactivation of Met in excitatory cortical neurons in mice (Emx1cre/Metflox mice) yields a phenotype in which significantly decreased GABAA receptor-mediated inhibition shifts the excitation/inhibition (E/I) balance toward excitation in the somatosensory cortex. Further, unlike that seen in wild-type mice, insulin does not increase inhibition in the mutant cortex, suggesting that one of the consequences of kinase inactive Met gene could be desensitization of insulin receptors. To test this hypothesis, we investigated the effects of insulin receptor sensitizer, pioglitazone, on inhibition in the somatosensory thalamocortical circuitry.
Methods: We used whole-cell patch clamp electrophysiology and analyzed excitatory and inhibitory responses of cortical layer IV excitatory cells following stimulation of their thalamic input in thalamocortical pathway intact brain slices. We applied insulin alone and insulin + a thiazolidinedione, pioglitazone (PIO), to test the effects of sensitizing insulin receptors on inhibitory responses mediated by GABAA receptors in the somatosensory cortex of Emx1cre/Metflox mice.
Results: In WT brain slices, application of insulin together with PIO did not enhance the effect of insulin alone. In contrast, PIO application induced a much larger inhibition than that of insulin alone in Met-defective cortex. Thus, insulin resistance of GABAA receptor-mediated response in Met mutant mice may result from desensitized insulin receptors.
Conclusions: Sporadic clinical studies reported improved behavioral symptoms in children with autism following PIO treatment. We show that PIO can aid in normalization of the E/I balance in the primary somatosensory cortex, a potential physiological mechanism underlying the positive effects of PIO treatment.
Keywords: Barrel cortex; GABAA receptors; Homeostatic plasticity; Met receptor tyrosine kinase; Pioglitazone; Thalamocortical circuitry.
Conflict of interest statement
All protocols strictly conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals (ISBN:13:978-0-309-15400-0, revised in 2011) and were approved by the University of Maryland Baltimore Institutional Animal Care and Use Committee.Not applicableThe authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

