Genome-wide association studies in Crohn's disease: Past, present and future
- PMID: 29484179
- PMCID: PMC5822399
- DOI: 10.1002/cti2.1001
Genome-wide association studies in Crohn's disease: Past, present and future
Abstract
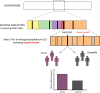
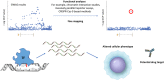
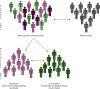
Over the course of the past decade, genome-wide association studies (GWAS) have revolutionised our understanding of complex disease genetics. One of the diseases that has benefitted most from this technology has been Crohn's disease (CD), with the identification of autophagy, the IL-17/IL-23 axis and innate lymphoid cells as key players in CD pathogenesis. Our increasing understanding of the genetic architecture of CD has also highlighted how a failure to suppress aberrant immune responses may contribute to disease development - a realisation that is now being incorporated into the design of new treatments. However, despite these successes, a significant proportion of disease heritability remains unexplained. Similarly, most of the causal variants at associated loci have not yet been identified, and even fewer have been functionally characterised. Because of the inarguable rise in the incidence of CD in regions of the world that previously had low disease rates, GWAS studies will soon have to shift from a largely Caucasian focus to include populations from other ethnic backgrounds. Future studies should also move beyond conventional studies of disease susceptibility into phenotypically driven 'within-cases' analyses in order to explore the role of genetics in other important aspects of disease biology. These studies are likely to include assessments of prognosis and/or response to treatments and may be critical if personalised medicine is ever to become a reality.
Keywords: Crohn's disease; GWAS; Pharmacogenetics; prognosis; susceptibility.
Figures
References
-
- Umicevic Mirkov M, Verstockt B, Cleynen I. Genetics of inflammatory bowel disease: beyond NOD2. Lancet Gastroenterol Hepatol 2017; 2: 224–234. - PubMed
-
- Hugot JP, Laurent‐Puig P, Gower‐Rousseau C et al Mapping of a susceptibility locus for Crohn's disease on chromosome 16. Nature 1996; 379: 821–823. - PubMed
-
- Hugot JP, Chamaillard M, Zouali H et al Association of NOD2 leucine‐rich repeat variants with susceptibility to Crohn's disease. Nature 2001; 411: 599–603. - PubMed
-
- Ogura Y, Bonen DK, Inohara N et al A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 2001; 411: 603–606. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
