Biology of Vascular Endothelial Growth Factor C in the Morphogenesis of Lymphatic Vessels
- PMID: 29484295
- PMCID: PMC5816233
- DOI: 10.3389/fbioe.2018.00007
Biology of Vascular Endothelial Growth Factor C in the Morphogenesis of Lymphatic Vessels
Abstract
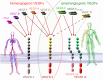
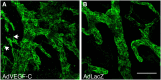
Because virtually all tissues contain blood vessels, the importance of hemevascularization has been long recognized in regenerative medicine and tissue engineering. However, the lymphatic vasculature has only recently become a subject of interest. Central to the task of growing a lymphatic network are lymphatic endothelial cells (LECs), which constitute the innermost layer of all lymphatic vessels. The central molecule that directs proliferation and migration of LECs during embryogenesis is vascular endothelial growth factor C (VEGF-C). VEGF-C is therefore an important ingredient for LEC culture and attempts to (re)generate lymphatic vessels and networks. During its biosynthesis VEGF-C undergoes a stepwise proteolytic processing, during which its properties and affinities for its interaction partners change. Many of these fundamental aspects of VEGF-C biosynthesis have only recently been uncovered. So far, most-if not all-applications of VEGF-C do not discriminate between different forms of VEGF-C. However, for lymphatic regeneration and engineering purposes, it appears mandatory to understand these differences, since they relate, e.g., to important aspects such as biodistribution and receptor activation potential. In this review, we discuss the molecular biology of VEGF-C as it relates to the growth of LECs and lymphatic vessels. However, the properties of VEGF-C are similarly relevant for the cardiovascular system, since both old and recent data show that VEGF-C can have a profound effect on the blood vasculature.
Keywords: A disintegrin and metalloproteinase with thrombospondin motifs 3; VEGF receptors; collagen and calcium binding EGF domains 1; growth factors; lymphatic vessels; lymphedema; tissue engineering; vascular endothelial growth factor C.
Figures


References
-
- Achen M. G., Jeltsch M., Kukk E., Mäkinen T., Vitali A., Wilks A. F., et al. (1998). Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. U.S.A. 95, 548–553. 10.1073/pnas.95.2.548 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

