IgE-Selective Immunoadsorption for Severe Atopic Dermatitis
- PMID: 29484297
- PMCID: PMC5816268
- DOI: 10.3389/fmed.2018.00027
IgE-Selective Immunoadsorption for Severe Atopic Dermatitis
Abstract
Introduction: Recent reports proposed the application of immunoadsorption (IA) for patients with recalcitrant atopic dermatitis (AD) and high-serum IgE levels. However, experience with this novel treatment approach, especially with the newly available IgE-specific adsorber, is limited and recommendation for its use in clinical practice awaits evidence from more studies.
Materials and methods: Patients with severe AD (SCORAD ≥ 60) and total serum IgE levels ≥750 kU/L were included in this study. The treatment protocol consisted of two cycles of five consecutive treatments with IgE-selective IA 3 weeks apart.
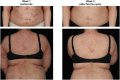
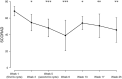
Results: Ten patients were enrolled and four patients completed the study. The mean SCORAD was significantly improved by up to 43% within a few weeks and until the end of a 6-month follow-up period, with 50% of patients achieving an at least 50% individual reduction of the baseline SCORAD. Each IA cycle induced a temporal average decrement of total serum levels of IgE, IgM, IgA, and IgG by 92, 43, 38, and 35%, respectively. Except for one case of Staphylococcus aureus septicemia, no major adverse events occurred.
Conclusion: Although limited by a considerable withdrawal rate, our observations strengthen our and other recent results further suggesting that IgE-selective IA is an effective treatment option for patients severely affected by AD with highly elevated IgE levels.
Keywords: IgE; SCORAD; atopic dermatitis; immunoadsorption; immunoglobulin.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

