AMP-activated protein kinase stimulates osteoblast differentiation and mineralization through autophagy induction
- PMID: 29484369
- PMCID: PMC5846672
- DOI: 10.3892/ijmm.2018.3498
AMP-activated protein kinase stimulates osteoblast differentiation and mineralization through autophagy induction
Abstract
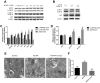
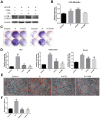
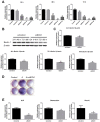
Previous studies have reported that adenosine monophosphate‑activated protein kinase (AMPK) activation can enhance osteoblast differentiation and mineralization; however, the underlying mechanism is not fully understood. Autophagy also serves an important role in osteoblast mineralization and bone homeostasis. The present study aimed to explore whether activation of AMPK could enhance osteoblast differentiation and mineralization via the induction of autophagy. The fracture healing and nonunion animal models were established and verified by X-ray imaging. Bone maturation was measured by Masson staining and the expression of AMPK, p-AMPK, microtubule-associated proteins 1A/1B light chain 3B II, and p62 in the fracture ends were detected by immunohistochemical staining. The mRNA expression levels of alkaline phosphatase (ALP), osteocalcin ,runt-related transcription factor 2 and BCN1 were determined by reverse transcription-quantitative polymerase chain reaction. 5-Bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium staining was used to determine ALP activity and alizarin red staining was adopted to examine mineralization. Western blot analysis was performed to detect protein expression. Autophagosome was observed by Transmission electron microscopy. Small interfering (si)RNA was used to knock down the expression of target gene. In vivo experiments demonstrated that new bone mineralization and maturation was markedly restrained in the nonunion group, alongside decreased AMPK activation and autophagic activity, compared with in the fracture healing group. The results of an in vitro study indicated that AMPK activation stimulated the osteogenic differentiation of MC3T3‑E1 cells, with increases in ALP activity, mineralization, and the mRNA expression levels of ALP, osteocalcin and runt-related transcription factor 2. Furthermore, AMPK activation induced autophagy, as determined by upregulation of microtubule‑associated proteins 1A/1B light chain 3B, increased autophagosome density and downregulation of p62. In addition, inhibition of autophagy reversed the effects of AMPK activation on osteoblast differentiation. These results suggested that AMPK activation may stimulate osteoblast differentiation and mineralization via the induction of autophagy, and provides evidence to suggest that enhancing AMPK activation and autophagic activity may be a potential novel approach to promote fracture healing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Vitamin K2 stimulates MC3T3‑E1 osteoblast differentiation and mineralization through autophagy induction.Mol Med Rep. 2019 May;19(5):3676-3684. doi: 10.3892/mmr.2019.10040. Epub 2019 Mar 15. Mol Med Rep. 2019. PMID: 30896842 Free PMC article.
-
IGF-I and IGFBP-2 Stimulate AMPK Activation and Autophagy, Which Are Required for Osteoblast Differentiation.Endocrinology. 2016 Jan;157(1):268-81. doi: 10.1210/en.2015-1690. Epub 2015 Nov 10. Endocrinology. 2016. PMID: 26556533 Free PMC article.
-
Osteoblast differentiation is functionally associated with decreased AMP kinase activity.J Cell Physiol. 2009 Dec;221(3):740-9. doi: 10.1002/jcp.21917. J Cell Physiol. 2009. PMID: 19725053
-
Implication and Regulation of AMPK during Physiological and Pathological Myeloid Differentiation.Int J Mol Sci. 2018 Sep 30;19(10):2991. doi: 10.3390/ijms19102991. Int J Mol Sci. 2018. PMID: 30274374 Free PMC article. Review.
-
AMP-activated protein kinase (AMPK) regulates autophagy, inflammation and immunity and contributes to osteoclast differentiation and functionabs.Biol Cell. 2020 Sep;112(9):251-264. doi: 10.1111/boc.202000008. Epub 2020 Jun 8. Biol Cell. 2020. PMID: 32445585 Review.
Cited by
-
Autophagy: An important target for natural products in the treatment of bone metabolic diseases.Front Pharmacol. 2022 Nov 18;13:999017. doi: 10.3389/fphar.2022.999017. eCollection 2022. Front Pharmacol. 2022. PMID: 36467069 Free PMC article. Review.
-
MiR-27a-3p Targets GLP1R to Regulate Differentiation, Autophagy, and Release of Inflammatory Factors in Pre-Osteoblasts via the AMPK Signaling Pathway.Front Genet. 2022 Jan 5;12:783352. doi: 10.3389/fgene.2021.783352. eCollection 2021. Front Genet. 2022. PMID: 35069685 Free PMC article.
-
Endocrinal metabolic regulation on the skeletal system in post-menopausal women.Front Physiol. 2022 Nov 11;13:1052429. doi: 10.3389/fphys.2022.1052429. eCollection 2022. Front Physiol. 2022. PMID: 36439254 Free PMC article. Review.
-
Autophagy Plays Multiple Roles in the Soft-Tissue Healing and Osseointegration in Dental Implant Surgery-A Narrative Review.Materials (Basel). 2022 Sep 1;15(17):6041. doi: 10.3390/ma15176041. Materials (Basel). 2022. PMID: 36079421 Free PMC article. Review.
-
Multiomics analysis of human serum and animal experiments reveals the protective mechanism of Qingre Huoxue Decoction against rheumatoid arthritis.Front Immunol. 2025 Mar 7;16:1526110. doi: 10.3389/fimmu.2025.1526110. eCollection 2025. Front Immunol. 2025. PMID: 40124380 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

