Dipeptidyl Peptidase 1 Inhibitor AZD7986 Induces a Sustained, Exposure-Dependent Reduction in Neutrophil Elastase Activity in Healthy Subjects
- PMID: 29484635
- PMCID: PMC6282495
- DOI: 10.1002/cpt.1053
Dipeptidyl Peptidase 1 Inhibitor AZD7986 Induces a Sustained, Exposure-Dependent Reduction in Neutrophil Elastase Activity in Healthy Subjects
Abstract
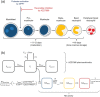
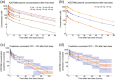
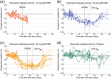
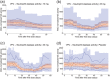
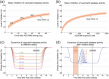
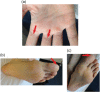
Neutrophil serine proteases (NSPs), such as neutrophil elastase (NE), are activated by dipeptidyl peptidase 1 (DPP1) during neutrophil maturation. High NSP levels can be detrimental, particularly in lung tissue, and inhibition of NSPs is therefore an interesting therapeutic opportunity in multiple lung diseases, including chronic obstructive pulmonary disease (COPD) and bronchiectasis. We conducted a randomized, placebo-controlled, first-in-human study to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of the DPP1 inhibitor AZD7986 in healthy subjects. Pharmacokinetic and pharmacodynamic data were analyzed using nonlinear mixed effects modeling and showed that AZD7986 inhibits whole blood NE activity in an exposure-dependent, indirect manner-consistent with in vitro and preclinical predictions. Several dose-dependent, possibly DPP1-related, nonserious skin findings were observed, but these were not considered to prevent further clinical development. Overall, the study results provided confidence to progress AZD7986 to phase II and supported selection of a clinically relevant dose.
© 2018 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures
References
-
- Pham, C.T. , Ivanovich, J.L. , Raptis, S.Z. , Zehnbauer, B. & Ley, T.J. Papillon‐Lefevre syndrome: correlating the molecular, cellular, and clinical consequences of cathepsin C/dipeptidyl peptidase I deficiency in humans. J. Immunol. 173, 7277–7281 (2004). - PubMed
-
- Cowland, J.B. & Borregaard, N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 273, 11–28 (2016). - PubMed
-
- Dalgic, B. , Bukulmez, A. & Sari, S. Eponym: Papillon‐Lefevre syndrome. Eur. J. Pediatr. 170, 689–691 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

