The dual-gate model for pentameric ligand-gated ion channels activation and desensitization
- PMID: 29484660
- PMCID: PMC5978336
- DOI: 10.1113/JP275100
The dual-gate model for pentameric ligand-gated ion channels activation and desensitization
Abstract
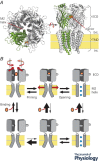
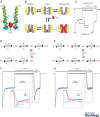
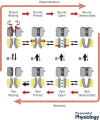
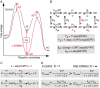
Pentameric ligand-gated ion channels (pLGICs) mediate fast neurotransmission in the nervous system. Their dysfunction is associated with psychiatric, neurological and neurodegenerative disorders such as schizophrenia, epilepsy and Alzheimer's disease. Understanding their biophysical and pharmacological properties, at both the functional and the structural level, thus holds many therapeutic promises. In addition to their agonist-elicited activation, most pLGICs display another key allosteric property, namely desensitization, in which they enter a shut state refractory to activation upon sustained agonist binding. While the activation mechanisms of several pLGICs have been revealed at near-atomic resolution, the structural foundation of desensitization has long remained elusive. Recent structural and functional data now suggest that the activation and desensitization gates are distinct, and are located at both sides of the ion channel. Such a 'dual gate mechanism' accounts for the marked allosteric effects of channel blockers, a feature illustrated herein by theoretical kinetics simulations. Comparison with other classes of ligand- and voltage-gated ion channels shows that this dual gate mechanism emerges as a common theme for the desensitization and inactivation properties of structurally unrelated ion channels.
Keywords: Allostery; Cys-loop receptors; GABA receptor; Glycine receptors; Inactivation; Nicotinic receptor; Pharmacology; Structure-function.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures








References
-
- Attwell D & Gibb A (2005). Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci 6, 841–849. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

