Parahydrogen-Based Hyperpolarization for Biomedicine
- PMID: 29484795
- PMCID: PMC6105405
- DOI: 10.1002/anie.201711842
Parahydrogen-Based Hyperpolarization for Biomedicine
Abstract
Magnetic resonance (MR) is one of the most versatile and useful physical effects used for human imaging, chemical analysis, and the elucidation of molecular structures. However, its full potential is rarely used, because only a small fraction of the nuclear spin ensemble is polarized, that is, aligned with the applied static magnetic field. Hyperpolarization methods seek other means to increase the polarization and thus the MR signal. A unique source of pure spin order is the entangled singlet spin state of dihydrogen, parahydrogen (pH2 ), which is inherently stable and long-lived. When brought into contact with another molecule, this "spin order on demand" allows the MR signal to be enhanced by several orders of magnitude. Considerable progress has been made in the past decade in the area of pH2 -based hyperpolarization techniques for biomedical applications. It is the goal of this Review to provide a selective overview of these developments, covering the areas of spin physics, catalysis, instrumentation, preparation of the contrast agents, and applications.
Keywords: NMR spectroscopy; hyperpolarization; magnetic resonance imaging; parahydrogen.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



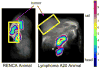

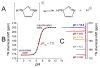
References
-
- Bowers CR, Weitekamp DP. Phys Rev Lett. 1986;57:2645–2648. - PubMed
- Bowers CR, Weitekamp DP. J Am Chem Soc. 1987;109:5541–5542.
- Pravica MG, Weitekamp DP. Chem Phys Lett. 1988;145:255–258.
-
- Eisenschmid TC, Kirss RU, Deutsch PP, Hommeltoft SI, Eisenberg R, Bargon J, Lawler RG, Balch AL. J Am Chem Soc. 1987;109:8089–8091.
-
- Reineri F, Boi T, Aime S. Nat Commun. 2015;6:5858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

