Biologically active quinoline and quinazoline alkaloids part II
- PMID: 29485730
- PMCID: PMC6105521
- DOI: 10.1002/med.21492
Biologically active quinoline and quinazoline alkaloids part II
Abstract
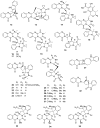
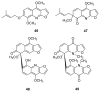
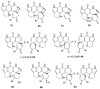
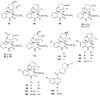
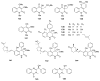
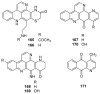
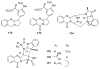
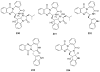
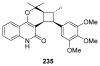
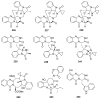
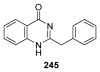
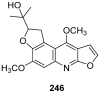
To follow-up on our prior Part I review, this Part II review summarizes and provides updated literature on novel quinoline and quinazoline alkaloids isolated during the period of 2009-2016, together with the biological activity and the mechanisms of action of these classes of natural products. Over 200 molecules with a broad range of biological activities, including antitumor, antiparasitic and insecticidal, antibacterial and antifungal, cardioprotective, antiviral, anti-inflammatory, hepatoprotective, antioxidant, anti-asthma, antitussive, and other activities, are discussed. This survey should provide new clues or possibilities for the discovery of new and better drugs from the original naturally occurring quinoline and quinazoline alkaloids.
Keywords: biological activities; camptothecin; natural products; quinazoline alkaloids; quinoline alkaloids.
© 2018 Wiley Periodicals, Inc.
Figures
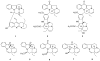
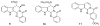
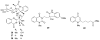
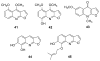

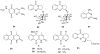
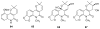
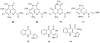
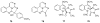
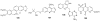
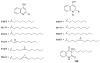
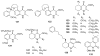
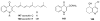
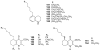
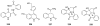
References
-
- Prajapati SM, Patel KD, Vekariya RH, Panchal SN, Patel HD. Recent advances in the synthesis of quinolines: a review. RSC Adv. 2014;4:24463–24476.
- Asif M. Chemical characteristics, synthetic methods, and biological potential of quinazoline and quinazolinone derivatives. Int J Med Chem. 2014;2014:395637. doi: 10.1155/2014/395637. - DOI - PMC - PubMed
- Rajput R, Mishra AP. A review on biological activity of quinazolinones. Int J Pharm Pharm Sci. 2012;4:66–70.
-
- Wiesner J, Ortmann R, Jomaa H, Schlitzer M. New antimalarial drugs. Angew Chem Int Ed. 2003;42:5274–5293. - PubMed
-
- Manske RH. The chemistry of quinolines. Chem Rev. 1942;30:113–144.
-
- Anjali PD, Singh D. Quinoline: a diverse therapeutic agent. Int J Pharm Sci Res. 2016;7:1–13.
-
- Afzal O, Kumar S, Haider MR, Ali MR, Kumar R, Jaggi M, Bawa S. A review on anticancer potential of bioactive heterocycle quinoline. Europ J Med Chem. 2015;97:871–910. - PubMed
- Selvan TP, Kumar PV. Quinazoline marketed drugs – a review. Res Pharmacy. 2011;1:1–21.
- Tiwary BK, Pradhan KP, Nanda AK, Chakraborty R. Implication of quinazoline-4(3H)-ones in medicinal chemistry: a brief review. J Chem Biol Ther. 2015;1 article 104.
- Patel DB, Vekariyz RH, Patel KD, Projapati NP, Vasava MS, Patel HD. Recent advances in synthesis of quinoline-4-carboxylic acid and their biological evaluation: a review. J Chem Pharm Res. 2017;9:216–230.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

