Redirection to the bone marrow improves T cell persistence and antitumor functions
- PMID: 29485974
- PMCID: PMC5919805
- DOI: 10.1172/JCI97454
Redirection to the bone marrow improves T cell persistence and antitumor functions
Abstract
A key predictor for the success of gene-modified T cell therapies for cancer is the persistence of transferred cells in the patient. The propensity of less differentiated memory T cells to expand and survive efficiently has therefore made them attractive candidates for clinical application. We hypothesized that redirecting T cells to specialized niches in the BM that support memory differentiation would confer increased therapeutic efficacy. We show that overexpression of chemokine receptor CXCR4 in CD8+ T cells (TCXCR4) enhanced their migration toward vascular-associated CXCL12+ cells in the BM and increased their local engraftment. Increased access of TCXCR4 to the BM microenvironment induced IL-15-dependent homeostatic expansion and promoted the differentiation of memory precursor-like cells with low expression of programmed death-1, resistance to apoptosis, and a heightened capacity to generate polyfunctional cytokine-producing effector cells. Following transfer to lymphoma-bearing mice, TCXCR4 showed a greater capacity for effector expansion and better tumor protection, the latter being independent of changes in trafficking to the tumor bed or local out-competition of regulatory T cells. Thus, redirected homing of T cells to the BM confers increased memory differentiation and antitumor immunity, suggesting an innovative solution to increase the persistence and functions of therapeutic T cells.
Keywords: Cancer immunotherapy; Chemokines; Immunology; T cells; Therapeutics.
Conflict of interest statement
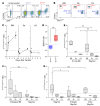
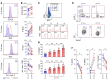
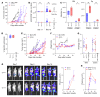
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

