Hydrogen Sulfide Attenuates Hypertensive Inflammation via Regulating Connexin Expression in Spontaneously Hypertensive Rats
- PMID: 29485979
- PMCID: PMC5841927
- DOI: 10.12659/msm.908761
Hydrogen Sulfide Attenuates Hypertensive Inflammation via Regulating Connexin Expression in Spontaneously Hypertensive Rats
Abstract
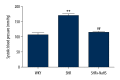
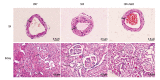
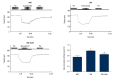
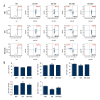
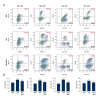
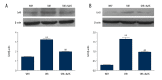
BACKGROUND Hydrogen sulfide (H2S) has anti-inflammatory and anti-hypertensive effects, and connexins (Cxs) are involved in regulation of immune homeostasis. In this study, we explored whether exogenous H2S prevents hypertensive inflammation by regulating Cxs expression of T lymphocytes in spontaneously hypertensive rats (SHR). MATERIAL AND METHODS We treated SHR with sodium hydrosulfide (NaHS) for 9 weeks. Vehicle-treated Wistar-Kyoto rats (WKYs) were used as a control. The arterial pressure was monitored by the tail-cuff method, and vascular function in basilar arteries was examined by pressure myography. Hematoxylin and eosin staining was used to show vascular remodeling and renal injury. The percentage of T cell subtypes in peripheral blood, surface expressions of Cx40/Cx43 on T cell subtypes, and serum cytokines level were determined by flow cytometry or ELISA. Expression of Cx40/Cx43 proteins in peripheral blood lymphocytes was analyzed by Western blot. RESULTS Chronic NaHS treatment significantly attenuated blood pressure elevation, and inhibited inflammation of target organs, vascular remodeling, and renal injury in SHR. Exogenous NaHS also improved vascular function by attenuating KCl-stimulated vasoconstrictor response in basilar arteries of SHR. In addition, chronic NaHS administration significantly suppressed inflammation of peripheral blood in SHR, as evidenced by the decreased serum levels of IL-2, IL-6, and CD4/CD8 ratio and the increased IL-10 level and percentage of regulatory T cells. NaHS treatment decreased hypertension-induced Cx40/Cx43 expressions in T lymphocytes from SHR. CONCLUSIONS Our data demonstrate that H2S reduces hypertensive inflammation, at least partly due to regulation of T cell subsets balance by Cx40/Cx43 expressions inhibition.
Conflict of interest statement
None.
Figures
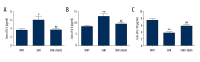
Similar articles
-
β‑estradiol alleviates hypertension‑ and concanavalin A‑mediated inflammatory responses via modulation of connexins in peripheral blood lymphocytes.Mol Med Rep. 2019 May;19(5):3743-3755. doi: 10.3892/mmr.2019.10037. Epub 2019 Mar 14. Mol Med Rep. 2019. PMID: 30896818 Free PMC article.
-
Increased expression and functionality of the gap junction in peripheral blood lymphocytes is associated with hypertension-mediated inflammation in spontaneously hypertensive rats.Cell Mol Biol Lett. 2018 Aug 20;23:40. doi: 10.1186/s11658-018-0106-0. eCollection 2018. Cell Mol Biol Lett. 2018. PMID: 30151015 Free PMC article.
-
Exogenous hydrogen sulfide (H2S) reduces blood pressure and prevents the progression of diabetic nephropathy in spontaneously hypertensive rats.Ren Fail. 2012;34(2):203-10. doi: 10.3109/0886022X.2011.643365. Epub 2012 Jan 9. Ren Fail. 2012. PMID: 22229751
-
The role of hydrogen sulphide in blood pressure regulation.Physiol Res. 2016 Oct 24;65(Suppl 3):S273-S289. doi: 10.33549/physiolres.933438. Physiol Res. 2016. PMID: 27775417 Review.
-
Mitogen-activated protein kinase cascade, endothelial function and vasomotor tone in the spontaneously hypertensive rat.J Hypertens. 2002 Jun;20(6):1053-4. doi: 10.1097/00004872-200206000-00007. J Hypertens. 2002. PMID: 12023665 Review. No abstract available.
Cited by
-
Central Administration of Hydrogen Sulfide Donor NaHS Reduces Iba1-Positive Cells in the PVN and Attenuates Rodent Angiotensin II Hypertension.Front Neurosci. 2021 Sep 13;15:690919. doi: 10.3389/fnins.2021.690919. eCollection 2021. Front Neurosci. 2021. PMID: 34602965 Free PMC article.
-
A Working Hypothesis Regarding Identical Pathomechanisms between Clinical Efficacy and Adverse Reaction of Clozapine via the Activation of Connexin43.Int J Mol Sci. 2020 Sep 24;21(19):7019. doi: 10.3390/ijms21197019. Int J Mol Sci. 2020. PMID: 32987640 Free PMC article. Review.
-
Estrogen Protects against Renal Ischemia-Reperfusion Injury by Regulating Th17/Treg Cell Immune Balance.Dis Markers. 2022 Oct 6;2022:7812099. doi: 10.1155/2022/7812099. eCollection 2022. Dis Markers. 2022. PMID: 36246554 Free PMC article.
-
Hydrogen sulfide alleviates lipopolysaccharide-induced myocardial injury through TLR4-NLRP3 pathway.Physiol Res. 2023 Mar 8;72(1):15-25. doi: 10.33549/physiolres.934928. Epub 2022 Dec 22. Physiol Res. 2023. PMID: 36545872 Free PMC article.
-
Sodium Thiosulphate-Loaded Liposomes Control Hydrogen Sulphide Release and Retain Its Biological Properties in Hypoxia-like Environment.Antioxidants (Basel). 2022 Oct 24;11(11):2092. doi: 10.3390/antiox11112092. Antioxidants (Basel). 2022. PMID: 36358464 Free PMC article.
References
-
- Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 2014;126(4):267–74. - PubMed
-
- Leibowitz A, Schiffrin EL. Immune mechanisms in hypertension. Curr Hypertens Rep. 2011;13(6):465–72. - PubMed
-
- Virdis A, Dell’Agnello U, Taddei S. Impact of inflammation on vascular disease in hypertension. Maturitas. 2014;78(3):179–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

