Severe hypoglycemia-induced sudden death is mediated by both cardiac arrhythmias and seizures
- PMID: 29486140
- PMCID: PMC6139495
- DOI: 10.1152/ajpendo.00442.2017
Severe hypoglycemia-induced sudden death is mediated by both cardiac arrhythmias and seizures
Abstract
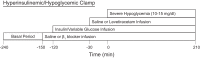
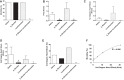
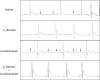
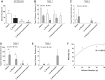
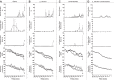
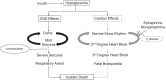
We previously demonstrated that insulin-induced severe hypoglycemia-associated sudden death is largely mediated by fatal cardiac arrhythmias. In the current study, a pharmacological approach was taken to explore the potential contribution of hypoglycemic seizures and the sympathoadrenergic system in mediating severe hypoglycemia-associated sudden death. Adult Sprague-Dawley rats were randomized into one of four treatment groups: 1) saline (SAL), 2) anti-arrhythmic (β1 blocker atenolol), 3) antiseizure (levetiracetam), and 4) combination antiarrhythmic and antiseizure (β1 Blocker+Levetiracetam). All rats underwent hyperinsulinemic severe hypoglycemic clamps for 3.5 h. When administered individually during severe hypoglycemia, β1 blocker reduced 2nd and 3rd degree heart block by 7.7- and 1.6-fold, respectively, and levetiracetam reduced seizures 2.7-fold, but mortality in these groups did not decrease. However, it was combined treatment with both β1 blocker and levetiracetam that remarkably reduced seizures and completely prevented respiratory arrest, while also eliminating 2nd and 3rd degree heart block, leading to 100% survival. These novel findings demonstrate that, in mediating sudden death, hypoglycemia elicits two distinct pathways (seizure-associated respiratory arrest and arrhythmia-associated cardiac arrest), and therefore, prevention of both seizures and cardiac arrhythmias is necessary to prevent severe hypoglycemia-induced mortality.
Keywords: cardiac arrhythmias; diabetes; hypoglycemia; seizures; sudden death.
Figures

References
-
- Chan O, Cheng H, Herzog R, Czyzyk D, Zhu W, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia. Diabetes 57: 1363–1370, 2008. doi: 10.2337/db07-1559. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

