Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish
- PMID: 29486195
- PMCID: PMC5830170
- DOI: 10.1016/j.devcel.2018.01.021
Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish
Abstract
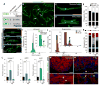
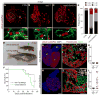
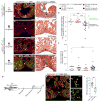
Correlative evidence suggests that polyploidization of heart muscle, which occurs naturally in post-natal mammals, creates a barrier to heart regeneration. Here, we move beyond a correlation by demonstrating that experimental polyploidization of zebrafish cardiomyocytes is sufficient to suppress their proliferative potential during regeneration. Initially, we determined that zebrafish myocardium becomes susceptible to polyploidization upon transient cytokinesis inhibition mediated by dominant-negative Ect2. Using a transgenic strategy, we generated adult animals containing mosaic hearts composed of differentially labeled diploid and polyploid-enriched cardiomyocyte populations. Diploid cardiomyocytes outcompeted their polyploid neighbors in producing regenerated heart muscle. Moreover, hearts composed of equivalent proportions of diploid and polyploid cardiomyocytes failed to regenerate altogether, demonstrating that a critical percentage of diploid cardiomyocytes is required to achieve heart regeneration. Our data identify cardiomyocyte polyploidization as a barrier to heart regeneration and suggest that mobilizing rare diploid cardiomyocytes in the human heart will improve its regenerative capacity.
Keywords: cardiomyocyte; cardiomyocyte proliferation; heart regeneration; polyploidization; zebrafish.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
A Role for Ploidy in Heart Regeneration.Dev Cell. 2018 Feb 26;44(4):403-404. doi: 10.1016/j.devcel.2018.02.004. Dev Cell. 2018. PMID: 29486188
-
Regeneration: Mending broken hearts.Nat Rev Mol Cell Biol. 2018 May;19(5):277. doi: 10.1038/nrm.2018.18. Epub 2018 Mar 7. Nat Rev Mol Cell Biol. 2018. PMID: 29511343 No abstract available.
-
Regeneration: Mending broken hearts.Nat Rev Cardiol. 2018 May;15(5):253. doi: 10.1038/nrcardio.2018.30. Epub 2018 Mar 22. Nat Rev Cardiol. 2018. PMID: 29565034 No abstract available.
References
-
- Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, Udi Y, Sarig R, Sagi I, Martin JF, Bursac N, Cohen S, Tzahor E. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547:179–184. doi: 10.1038/nature22978. - DOI - PMC - PubMed
-
- Becker RO, Chapin S, Sherry R. Regeneration of the ventricular myocardium in amphibians. Nature. 1974;248:145–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

