Effect of storage temperature on the stability of spray dried bacteriophage powders
- PMID: 29486303
- PMCID: PMC5948144
- DOI: 10.1016/j.ejpb.2018.02.033
Effect of storage temperature on the stability of spray dried bacteriophage powders
Abstract
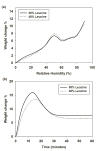
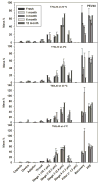
This study aimed to assess the robustness of using a spray drying approach and formulation design in producing inhalable phage powders. Two types of Pseudomonas phages, PEV2 (Podovirus) and PEV40 (Myovirus) in two formulations containing different amounts of trehalose (70% and 60%) and leucine (30% and 40%) were studied. Most of the surface of the produced powders was found to be covered in crystalline leucine. The powders were stored at 4 °C and 20 °C under vacuum. The phage stability and in vitro aerosol performance of the phage powders were examined on the day of production and after 1, 3 and 12 months of storage. A minor titer loss during production was observed for both phages (0.2-0.8 log10 pfu/ml). The storage stability of the produced phage powders was found to be phage and formulation dependent. No further reduction in titer occurred for PEV2 powders stored at 4 °C across the study. The formulation containing 30% leucine maintained the viability of PEV2 at 20 °C, while the formulation containing 40% leucine gradually lost titer over time with a storage reduction of ∼0.9 log10 pfu/ml measured after 12 months. In comparison, the PEV40 phage powders generally had a ∼ 0.5 log10 pfu/ml loss upon storage regardless of temperature. When aerosolized, the total in vitro lung doses of PEV2 were of the order of 107 pfu, except the formulation containing 40% leucine stored at 20 °C which had a lower lung dose. The PEV40 powders also had lung doses of 106-107 pfu. The results demonstrate that spray dried Myoviridae and Podoviridae phage in a simple formulation of leucine and trehalose can be successfully stored for one year at 4 °C and 20 °C with vacuum packaging.
Keywords: Antibiotic resistance; PEV2; PEV40; Phage; Phage dry powder; Pulmonary infections.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures





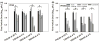
References
-
- Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, Balloy V, Touqui L. Bacteriophages Can Treat and Prevent Pseudomonas aeruginosa Lung Infections. Journal of Infectious Diseases. 2010;201:1096–1104. - PubMed
-
- Sahota JS, Smith CM, Radhakrishnan P, Winstanley C, Goderdzishvili M, Chanishvili N, Kadioglu A, O'Callaghan C, Clokie MR. Bacteriophage Delivery by Nebulization and Efficacy Against Phenotypically Diverse Pseudomonas aeruginosa from Cystic Fibrosis Patients. J Aerosol Med Pulm Drug Deliv. 2015;28:353–360. - PubMed
-
- Saussereau E, Vachier I, Chiron R, Godbert B, Sermet I, Dufour N, Pirnay JP, De Vos D, Carrie F, Molinari N, Debarbieux L. Effectiveness of bacteriophages in the sputum of cystic fibrosis patients. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20:O983–90. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

