The impact of in-scanner head motion on structural connectivity derived from diffusion MRI
- PMID: 29486323
- PMCID: PMC5911236
- DOI: 10.1016/j.neuroimage.2018.02.041
The impact of in-scanner head motion on structural connectivity derived from diffusion MRI
Abstract
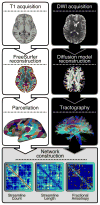
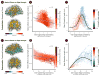
Multiple studies have shown that data quality is a critical confound in the construction of brain networks derived from functional MRI. This problem is particularly relevant for studies of human brain development where important variables (such as participant age) are correlated with data quality. Nevertheless, the impact of head motion on estimates of structural connectivity derived from diffusion tractography methods remains poorly characterized. Here, we evaluated the impact of in-scanner head motion on structural connectivity using a sample of 949 participants (ages 8-23 years old) who passed a rigorous quality assessment protocol for diffusion magnetic resonance imaging (dMRI) acquired as part of the Philadelphia Neurodevelopmental Cohort. Structural brain networks were constructed for each participant using both deterministic and probabilistic tractography. We hypothesized that subtle variation in head motion would systematically bias estimates of structural connectivity and confound developmental inference, as observed in previous studies of functional connectivity. Even following quality assurance and retrospective correction for head motion, eddy currents, and field distortions, in-scanner head motion significantly impacted the strength of structural connectivity in a consistency- and length-dependent manner. Specifically, increased head motion was associated with reduced estimates of structural connectivity for network edges with high inter-subject consistency, which included both short- and long-range connections. In contrast, motion inflated estimates of structural connectivity for low-consistency network edges that were primarily shorter-range. Finally, we demonstrate that age-related differences in head motion can both inflate and obscure developmental inferences on structural connectivity. Taken together, these data delineate the systematic impact of head motion on structural connectivity, and provide a critical context for identifying motion-related confounds in studies of structural brain network development.
Keywords: Artifact; Confound; DTI; Development; Motion; Structural connectivity; dMRI.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


References
-
- Aksoy M, Forman C, Straka M, Skare S, Holdsworth S, Hornegger J, Bammer R. Real-time Optical Motion Correction for Diffusion Tensor Imaging. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2011;66:366–378. https://doi.org/10.1002/mrm.22787. - DOI - PMC - PubMed
-
- Aksoy M, Liu C, Moseley ME, Bammer R. Single-Step Nonlinear Diffusion Tensor Estimation in the Presence of Microscopic and Macroscopic Motion. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2008;59:1138–1150. https://doi.org/10.1002/mrm.21558. - DOI - PMC - PubMed
-
- Alexander DC, Dyrby TB, Nilsson M, Zhang H. Imaging brain microstructure with diffusion MRI: practicality and applications. NMR Biomed. 2017 https://doi.org/10.1002/nbm.3841. - DOI - PubMed
-
- Alexander-Bloch A, Clasen L, Stockman M, Ronan L, Lalonde F, Giedd J, Raznahan A. Subtle in-scanner motion biases automated measurement of brain anatomy from in vivo MRI. Hum Brain Mapp. 2016;37:2385–2397. https://doi.org/10.1002/hbm.23180. - DOI - PMC - PubMed
-
- Alhamud A, Taylor PA, Laughton B, van der Kouwe AJW, Meintjes EM. Motion artifact reduction in pediatric diffusion tensor imaging using fast prospective correction. J Magn Reson Imaging JMRI. 2015;41:1353–1364. https://doi.org/10.1002/jmri.24678. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS099348/NS/NINDS NIH HHS/United States
- R01 MH107235/MH/NIMH NIH HHS/United States
- R01 DC009209/DC/NIDCD NIH HHS/United States
- RC2 MH089924/MH/NIMH NIH HHS/United States
- R01 MH109520/MH/NIMH NIH HHS/United States
- R01 NS085211/NS/NINDS NIH HHS/United States
- P50 MH096891/MH/NIMH NIH HHS/United States
- R01 MH112847/MH/NIMH NIH HHS/United States
- R21 MH106799/MH/NIMH NIH HHS/United States
- R01 MH112070/MH/NIMH NIH HHS/United States
- K01 MH102609/MH/NIMH NIH HHS/United States
- R01 HD086888/HD/NICHD NIH HHS/United States
- R01 MH107703/MH/NIMH NIH HHS/United States
- RC2 MH089983/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

