Development and evaluation of a risk assessment tool to improve clinical triage accuracy for colonoscopic investigations
- PMID: 29486733
- PMCID: PMC6389276
- DOI: 10.1186/s12885-018-4140-0
Development and evaluation of a risk assessment tool to improve clinical triage accuracy for colonoscopic investigations
Abstract
Background: Gastroenterology Departments at hospitals within Australia receive thousands of General Practitioner (GP)-referral letters for gastrointestinal investigations every month. Many of these requests are for colonoscopy. This study aims to evaluate the performance of the current symptoms-based triage system compared to a novel risk score using objective markers.
Methods: Patients with lower abdominal symptoms referred by their GPs and triaged by a Gastroenterology consultant to a colonoscopy consent clinic were recruited into the study. A risk assessment tool (RAT) was developed using objective data (clinical, demographic, pathology (stool test, FIT), standard blood tests and colonoscopy outcome). Colonoscopy and histology results were scored and then stratified as either significant bowel disease (SBD) or non-significant bowel disease (non-SBD).
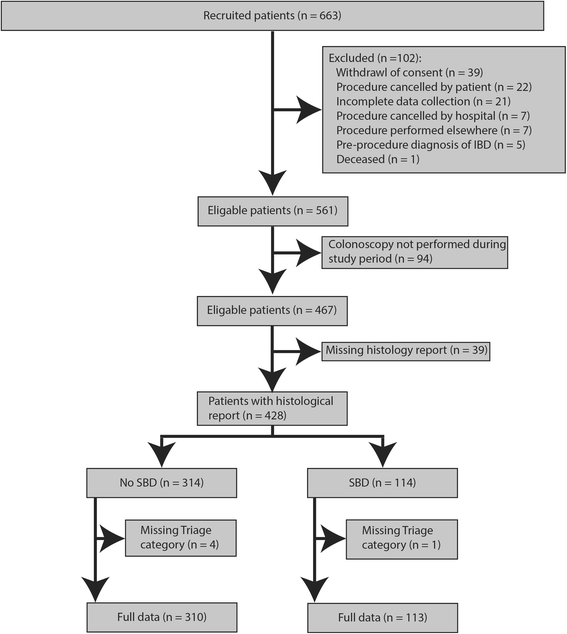
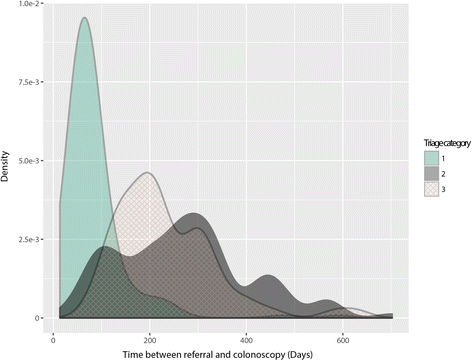
Results: Of the 467 patients in our study, 45.1% were male, the mean age was 54.3 ± 13.8 years and mean BMI was 27.8 ± 6.2. Overall, 26% had SBD compared to 74% with non-SBD (42% of the cohort had a normal colonoscopy). Increasing severity of referral symptoms was related to a higher triage category, (rectal bleeding, P = 2.86*10-9; diarrhoea, P = 0.026; abdominal pain, P = 5.67*10-4). However, there was no significant difference in the prevalence of rectal bleeding (P = 0.991) or diarrhoea (P = 0.843) for SBD. Abdominal pain significantly reduced the risk of SBD (P = 0.0344, OR = 0.52, CI = 0.27-0.95). Conversely, the RAT had a very high specificity of 98% with PPV and NPV of SBD prediction, 74% and 77%, respectively. The RAT provided an odds ratio (OR) of 9.0, 95%CI 4.29-18.75, p = 2.32*10-11), higher than the FIT test (OR = 5.3, 95%CI 2.44-11.69, p = 4.88*10-6), blood score (OR = 2.8, 95%CI 1.72- 4.38, p = 1.47*10-5) or age (OR = 2.5, 95%CI 1.61-4.00, 5.12*10-5) independently. Notably, the ORs of these individual objective measures were higher than the current practice of symptoms-based triaging (OR = 1.4, 95%CI 0.88-2.11, p = 0.153).
Conclusions: It is critical that individuals with high risk of having SBD are triaged to the appropriate category with the shortest wait time. Here we provide evidence that a combination of blood markers, demographic markers and the FIT test have a higher diagnostic accuracy for SBD than FIT alone.
Keywords: Bowel disease; Colonoscopy; Colorectal cancer; Faecal immunochemical test; Risk assessment tool.
Conflict of interest statement
Ethics approval and consent to participate
The Human Research Ethics Committee of The Prince Charles Hospital approved the study (protocol: HREC/13/QPCH/47). All participants gave written informed consent before enrolment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Australian Institute of Health and Welfare and the Australian Government Department of Health. Analysis of colorectal cancer outcomes for the Australian National Bowel Cancer Screening Program. Asia Pac J Clin Oncol. 2016;12(1):22-32. - PubMed
-
- Department of Health AG . Public release of linkable 10% sample of Medicare benefits scheme (Medicare) and pharmaceutical benefits scheme (PBS) data. 2016.
-
- Welfare and the Australian Government Department of Health. National Bowel Cancer Screening Program: Monitoring Report [press release]. 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

