Cross-communication between Gi and Gs in a G-protein-coupled receptor heterotetramer guided by a receptor C-terminal domain
- PMID: 29486745
- PMCID: PMC6389107
- DOI: 10.1186/s12915-018-0491-x
Cross-communication between Gi and Gs in a G-protein-coupled receptor heterotetramer guided by a receptor C-terminal domain
Abstract
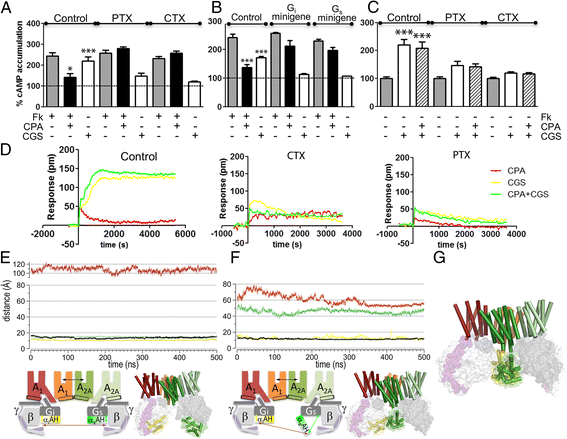
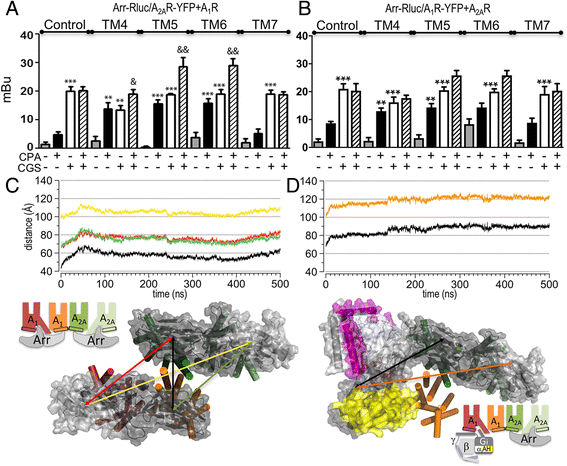
Background: G-protein-coupled receptor (GPCR) heteromeric complexes have distinct properties from homomeric GPCRs, giving rise to new receptor functionalities. Adenosine receptors (A1R or A2AR) can form A1R-A2AR heteromers (A1-A2AHet), and their activation leads to canonical G-protein-dependent (adenylate cyclase mediated) and -independent (β-arrestin mediated) signaling. Adenosine has different affinities for A1R and A2AR, allowing the heteromeric receptor to detect its concentration by integrating the downstream Gi- and Gs-dependent signals. cAMP accumulation and β-arrestin recruitment assays have shown that, within the complex, activation of A2AR impedes signaling via A1R.
Results: We examined the mechanism by which A1-A2AHet integrates Gi- and Gs-dependent signals. A1R blockade by A2AR in the A1-A2AHet is not observed in the absence of A2AR activation by agonists, in the absence of the C-terminal domain of A2AR, or in the presence of synthetic peptides that disrupt the heteromer interface of A1-A2AHet, indicating that signaling mediated by A1R and A2AR is controlled by both Gi and Gs proteins.
Conclusions: We identified a new mechanism of signal transduction that implies a cross-communication between Gi and Gs proteins guided by the C-terminal tail of the A2AR. This mechanism provides the molecular basis for the operation of the A1-A2AHet as an adenosine concentration-sensing device that modulates the signals originating at both A1R and A2AR.
Keywords: BRET; C-terminal domain; GPCR; Heterotetramer; Molecular modeling.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

