Eltrombopag for use in children with immune thrombocytopenia
- PMID: 29487060
- PMCID: PMC5858473
- DOI: 10.1182/bloodadvances.2017010660
Eltrombopag for use in children with immune thrombocytopenia
Abstract
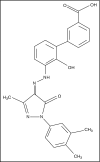
Eltrombopag is currently the only US Food and Drug Administration-approved thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia (ITP) in children. This oral, once-per-day therapy has shown favorable efficacy and adverse effect profiles in children. Two multicenter, double-blind, placebo controlled clinical trials (PETIT [Efficacy and Safety Study of Eltrombopag in Pediatric Patients With Thrombocytopenia From Chronic Idiopathic Thrombocytopenic Purpura (ITP)] and PETIT2 [Study of a New Medication for Childhood Chronic Immune Thrombocytopenia (ITP), a Blood Disorder of Low Platelet Counts That Can Lead to Bruising Easily, Bleeding Gums, and/or Bleeding Inside the Body]) demonstrated efficacy in raising platelet counts, reducing bleeding, and reducing the need for concomitant ITP therapies with relatively few adverse effects. The most commonly reported drug-related adverse effects include headache, nausea, and hepatobiliary laboratory abnormalities. Long-term safety data in children are limited, and studies in adults have not revealed a clinically significant increased incidence of thrombosis, marrow fibrosis, or cataract formation. Eltrombopag has also been approved for treating refractory severe aplastic anemia (AA) and has potential for expanded use in ITP and severe AA as well as in other conditions associated with thrombocytopenia.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.P.L. served on advisory boards for Novartis and Bayer and was a consultant for Novartis. J.D. was a consultant for Sanofi-Genzyme. T.O.K. declares no competing financial interests.
Figures



References
-
- Cserhati I, Kelemen E. Acute prolonged thrombocytosis in mice induced by thrombocythaemic sera; a possible human thrombopoietin; a preliminary communication. Acta Med Acad Sci Hung. 1958;11(4):473-475. - PubMed
-
- Bartley TD, Bogenberger J, Hunt P, et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994;77(7):1117-1124. - PubMed
-
- de Sauvage FJ, Hass PE, Spencer SD, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369(6481):533-538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

