Target selectivity of septal cholinergic neurons in the medial and lateral entorhinal cortex
- PMID: 29487212
- PMCID: PMC5856533
- DOI: 10.1073/pnas.1716531115
Target selectivity of septal cholinergic neurons in the medial and lateral entorhinal cortex
Abstract
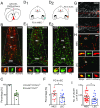
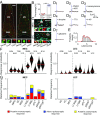
The entorhinal cortex (EC) plays a pivotal role in processing and conveying spatial information to the hippocampus. It has long been known that EC neurons are modulated by cholinergic input from the medial septum. However, little is known as to how synaptic release of acetylcholine affects the different cell types in EC. Here we combined optogenetics and patch-clamp recordings to study the effect of cholinergic axon stimulation on distinct neurons in EC. We found dense cholinergic innervations that terminate in layer I and II (LI and LII). Light-activated stimulation of septal cholinergic projections revealed differential responses in excitatory and inhibitory neurons in LI and LII of both medial and lateral EC. We observed depolarizing responses mediated by nicotinic and muscarinic receptors primarily in putative serotonin receptor (p5HT3R)-expressing interneurons. Hyperpolarizing muscarinic receptor-mediated responses were found predominantly in excitatory cells. Additionally, some excitatory as well as a higher fraction of inhibitory neurons received mono- and/or polysynaptic GABAergic inputs, revealing that medial septum cholinergic neurons have the capacity to corelease GABA alongside acetylcholine. Notably, the synaptic effects of acetylcholine were similar in neurons of both medial and lateral EC. Taken together, our findings demonstrate that EC activity may be differentially modulated via the activation or the suppression of distinct subsets of LI and LII neurons by the septal cholinergic system.
Keywords: medial septum; muscarinic receptor; neurotransmitter corelease; nicotinic receptor; optogenetics.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976;167:285–314. - PubMed
-
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AHM. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. - PubMed
-
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. - PubMed
-
- Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: The lateral and medial entorhinal areas. Hippocampus. 2007;17:697–708. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

