Calcium-Dependent Protein Kinase 5 Is Required for Release of Egress-Specific Organelles in Plasmodium falciparum
- PMID: 29487234
- PMCID: PMC5829822
- DOI: 10.1128/mBio.00130-18
Calcium-Dependent Protein Kinase 5 Is Required for Release of Egress-Specific Organelles in Plasmodium falciparum
Abstract
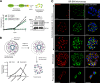
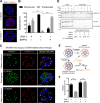
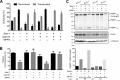
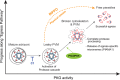
The human malaria parasite Plasmodium falciparum requires efficient egress out of an infected red blood cell for pathogenesis. This egress event is highly coordinated and is mediated by several signaling proteins, including the plant-like Pfalciparum calcium-dependent protein kinase 5 (PfCDPK5). Knockdown of PfCDPK5 results in an egress block where parasites are trapped inside their host cells. The mechanism of this PfCDPK5-dependent block, however, remains unknown. Here, we show that PfCDPK5 colocalizes with a specialized set of parasite organelles known as micronemes and is required for their discharge, implicating failure of this step as the cause of the egress defect in PfCDPK5-deficient parasites. Furthermore, we show that PfCDPK5 cooperates with the Pfalciparum cGMP-dependent kinase (PfPKG) to fully activate the protease cascade critical for parasite egress. The PfCDPK5-dependent arrest can be overcome by hyperactivation of PfPKG or by physical disruption of the arrested parasite, and we show that both treatments facilitate the release of the micronemes required for egress. Our results define the molecular mechanism of PfCDPK5 function and elucidate the complex signaling pathway of parasite egress.IMPORTANCE The signs and symptoms of clinical malaria result from the replication of parasites in human blood. Efficient egress of the malaria parasite Plasmodium falciparum out of an infected red blood cell is critical for pathogenesis. The Pfalciparum calcium-dependent protein kinase 5 (PfCDPK5) is essential for parasite egress. Following PfCDPK5 knockdown, parasites remain trapped inside their host cell and do not egress, but the mechanism for this block remains unknown. We show that PfCDPK5 colocalizes with parasite organelles known as micronemes. We demonstrate that PfCDPK5 is critical for the discharge of these micronemes and that failure of this step is the molecular mechanism of the parasite egress arrest. We also show that hyperactivation of the cGMP-dependent kinase PKG can overcome this arrest. Our data suggest that small molecules that inhibit the egress signaling pathway could be effective antimalarial therapeutics.
Keywords: Plasmodium falciparum; calcium-dependent protein kinase; egress; malaria; microneme.
Copyright © 2018 Absalon et al.
Figures


References
-
- World Health Organization 2016. World malaria report 2015. World Health Organization, Geneva, Switzerland.
-
- Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han K-T, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, et al. . 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi:10.1056/NEJMoa1314981. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

