Salmonella Reprograms Nucleotide Metabolism in Its Adaptation to Nitrosative Stress
- PMID: 29487237
- PMCID: PMC5829828
- DOI: 10.1128/mBio.00211-18
Salmonella Reprograms Nucleotide Metabolism in Its Adaptation to Nitrosative Stress
Abstract
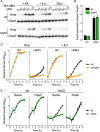
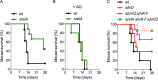
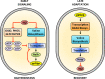
The adaptations that protect pathogenic microorganisms against the cytotoxicity of nitric oxide (NO) engendered in the immune response are incompletely understood. We show here that salmonellae experiencing nitrosative stress suffer dramatic losses of the nucleoside triphosphates ATP, GTP, CTP, and UTP while simultaneously generating a massive burst of the alarmone nucleotide guanosine tetraphosphate. RelA proteins associated with ribosomes overwhelmingly synthesize guanosine tetraphosphate in response to NO as a feedback mechanism to transient branched-chain amino acid auxotrophies. Guanosine tetraphosphate activates the transcription of valine biosynthetic genes, thereby reestablishing branched-chain amino acid biosynthesis that enables the translation of the NO-consuming flavohemoglobin Hmp. Guanosine tetraphosphate synthesized by RelA protects salmonellae from the metabolic stress inflicted by reactive nitrogen species generated in the mammalian host response. This research illustrates the importance of nucleotide metabolism in the adaptation of salmonellae to the nutritional stress imposed by NO released in the innate host response.IMPORTANCE Nitric oxide triggers dramatic drops in nucleoside triphosphates, the building blocks that power DNA replication; RNA transcription; translation; cell division; and the biosynthesis of fatty acids, lipopolysaccharide, and peptidoglycan. Concomitantly, this diatomic gas stimulates a burst of guanosine tetraphosphate. Global changes in nucleotide metabolism may contribute to the potent bacteriostatic activity of nitric oxide. In addition to inhibiting numerous growth-dependent processes, guanosine tetraphosphate positively regulates the transcription of branched-chain amino acid biosynthesis genes, thereby facilitating the translation of antinitrosative defenses that mediate recovery from nitrosative stress.
Keywords: Salmonella; adaptive resistance; animal models; cellular redox status; guanosine tetraphosphate; nitric oxide; nitrosative stress; nucleotide metabolism; stringent response.
Figures



References
-
- Schreiber F, Lynn DJ, Houston A, Peters J, Mwafulirwa G, Finlay BB, Brinkman FS, Hancock RE, Heyderman RS, Dougan G, Gordon MA. 2011. The human transcriptome during nontyphoid Salmonella and HIV coinfection reveals attenuated NFκB-mediated inflammation and persistent cell cycle disruption. J Infect Dis 204:1237–1245. doi: 10.1093/infdis/jir512. - DOI - PMC - PubMed
-
- Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, Rosenzweig SD, Newport M, Levin M, Roesler J, Kumararatne D, Casanova JL, Holland SM. 2004. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet 364:2113–2121. doi: 10.1016/S0140-6736(04)17552-1. - DOI - PubMed
-
- de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, Casanova JL, Ottenhoff TH. 1998. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280:1435–1438. doi: 10.1126/science.280.5368.1435. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
