Galectin-1 enhances TNFα-induced inflammatory responses in Sertoli cells through activation of MAPK signalling
- PMID: 29487346
- PMCID: PMC5829165
- DOI: 10.1038/s41598-018-22135-w
Galectin-1 enhances TNFα-induced inflammatory responses in Sertoli cells through activation of MAPK signalling
Abstract
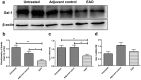
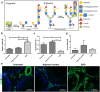
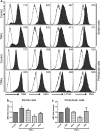
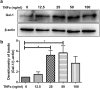
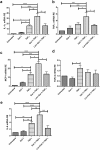
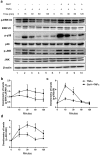
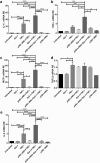
Galectin-1 (Gal-1) is a pleiotropic lectin involved in the modulation of immune responses. Using a model of rat experimental autoimmune orchitis (EAO), we investigated the role of Gal-1 in testicular inflammation. EAO is characterized by leukocytic infiltrates in the interstitium, damage of spermatogenesis and production of inflammatory mediators like TNFα and MCP1 causing infertility. In normal rat testis Gal-1 was mainly expressed in Sertoli cells and germ cells. In the inflamed testis, Gal-1 expression was significantly downregulated most likely due to germ cell loss. Analyses of lectin binding and expression of glucosaminyl- and sialyltransferases indicated that the glycan composition on the cell surface of Sertoli and peritubular cells becomes less favourable for Gal-1 binding under inflammatory conditions. In primary Sertoli cells Gal-1 expression was found to be upregulated after TNFα challenge. Pretreatment with Gal-1 synergistically and specifically enhanced TNFα-induced expression of MCP1, IL-1α, IL-6 and TNFα in Sertoli cells. Combined stimulation of Sertoli cells with Gal-1 and TNFα enhanced the phosphorylation of MAP kinases as compared to TNFα or Gal-1 alone. Taken together, our data show that Gal-1 modulates inflammatory responses in Sertoli cells by enhancing the pro-inflammatory activity of TNFα via stimulation of MAPK signalling.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Targeting high mobility group box protein 1 ameliorates testicular inflammation in experimental autoimmune orchitis.Hum Reprod. 2015 Feb;30(2):417-31. doi: 10.1093/humrep/deu320. Epub 2014 Dec 1. Hum Reprod. 2015. PMID: 25452436
-
Expression of galectin-3 and its regulation in the testes.Int J Androl. 2007 Feb;30(1):28-40. doi: 10.1111/j.1365-2605.2006.00707.x. Epub 2006 Nov 27. Int J Androl. 2007. PMID: 17132155
-
Investigation of activin A in inflammatory responses of the testis and its role in the development of testicular fibrosis.Hum Reprod. 2019 Aug 1;34(8):1536-1550. doi: 10.1093/humrep/dez109. Hum Reprod. 2019. PMID: 31340036
-
The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis.Reprod Biol Endocrinol. 2004 Mar 10;2:9. doi: 10.1186/1477-7827-2-9. Reprod Biol Endocrinol. 2004. PMID: 15012831 Free PMC article. Review.
-
Cytokines and chemokines in testicular inflammation: A brief review.Microsc Res Tech. 2009 Aug;72(8):620-8. doi: 10.1002/jemt.20704. Microsc Res Tech. 2009. PMID: 19263422 Review.
Cited by
-
Activin A and CCR2 regulate macrophage function in testicular fibrosis caused by experimental autoimmune orchitis.Cell Mol Life Sci. 2022 Nov 24;79(12):602. doi: 10.1007/s00018-022-04632-4. Cell Mol Life Sci. 2022. PMID: 36434305 Free PMC article.
-
Diabetes as a potential compounding factor in COVID-19-mediated male subfertility.Cell Biosci. 2022 Mar 20;12(1):35. doi: 10.1186/s13578-022-00766-x. Cell Biosci. 2022. PMID: 35307018 Free PMC article. Review.
-
Regulation of wound healing and fibrosis by galectins.J Mol Med (Berl). 2022 Jun;100(6):861-874. doi: 10.1007/s00109-022-02207-1. Epub 2022 May 19. J Mol Med (Berl). 2022. PMID: 35589840 Review.
-
Feasibility of Serum Galectin-1 as a Diagnostic Biomarker for Metabolic Dysfunction-Associated Steatotic Liver Disease: A Study on a Segment of the Chinese Population Using Convenience Sampling.Biomedicines. 2025 Feb 10;13(2):425. doi: 10.3390/biomedicines13020425. Biomedicines. 2025. PMID: 40002838 Free PMC article.
-
Multiple signaling pathways in Sertoli cells: recent findings in spermatogenesis.Cell Death Dis. 2019 Jul 17;10(8):541. doi: 10.1038/s41419-019-1782-z. Cell Death Dis. 2019. PMID: 31316051 Free PMC article. Review.
References
-
- Schuppe H. C.; Bergmann M. Inflammatory conditions of the testis. In: Atlas of the human testis. (Springer, 2013).
-
- Schuppe H-C, Meinhardt A. Immune privilege and inflammation of the testis. Chem. Immunol. Allergy. 2005;88:1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

