Antibodies to a strain-specific citrullinated Epstein-Barr virus peptide diagnoses rheumatoid arthritis
- PMID: 29487382
- PMCID: PMC5829227
- DOI: 10.1038/s41598-018-22058-6
Antibodies to a strain-specific citrullinated Epstein-Barr virus peptide diagnoses rheumatoid arthritis
Abstract
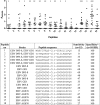
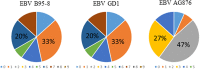
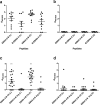
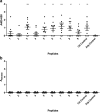
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease. Anti-citrullinated protein antibodies (ACPA) are crucial for the serological diagnosis of RA, where Epstein-Barr virus (EBV) has been suggested to be an environmental agent in triggering the onset of the disease. This study aimed to analyse antibody reactivity to citrullinated EBV nuclear antigen-2 (EBNA-2) peptides from three different EBV strains (B95-8, GD1 and AG876) using streptavidin capture enzyme-linked immunosorbent assay. One peptide, only found in a single strain (AG876), obtained a sensitivity and specificity of 77% and 95%, respectively and showed high sequence similarity to the filaggrin peptide originally used for ACPA detection. Comparison of antibody reactivity to commercial assays found that the citrullinated peptide was as effective in detecting ACPA as highly sensitive and specific commercial assays. The data presented demonstrate that the citrullinated EBNA-2 peptide indeed is recognised specifically by RA sera and that the single peptide is able to compete with assays containing multiple peptides. Furthermore, it could be hypothesized that RA may be caused by (a) specific strain(s) of EBV.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35–61. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

