Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes
- PMID: 29487386
- PMCID: PMC6916728
- DOI: 10.1038/s41375-018-0070-8
Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes
Abstract
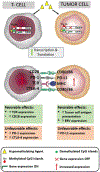
Immune checkpoint inhibitors, as single-agent therapy, have shown modest clinical efficacy in the treatment of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). As has been successfully shown in other less immunogenic hematologic malignancies, rationally designed combination approaches may be more effective than single-agent checkpoint inhibitors, and may be the approach to pursue in AML/MDS. Hypomethylating agents (HMAs) such as azacitidine, while enhancing anti-tumor immune response, concurrently dampen immune response by upregulating inhibitory immune checkpoint molecule expression. Immune checkpoint molecule upregulation may be an important mechanism of azacitidine resistance. These findings have resulted in multiple clinical trials combining HMAs with immune checkpoint blockade. Clinical trial data have shown encouraging response rates and durable responses without resorting to stem cell transplant. In this review, we discuss preclinical data supporting the use of these agents in combination, and focus on clinical and correlative data emerging from numerous clinical trials investigating HMA-immune checkpoint inhibitor combinations in AML/MDS.
Conflict of interest statement
Conflict of interest
ND has received research funding from BMS, Pfizer, Merck, and served as a consultant for BMS, Pfizer, and Celgene. HK has received research funding from BMS, Pfizer, and served as a consultant for Pfizer. PS and JA have served as consultants for BMS, EMD Serrono, and AstraZeneca. The remaining authors declare that they have no conflict of interest.
Figures

References
-
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

